在IVDR法規(guī)中�,伴隨診斷產(chǎn)品的定義如下【Article 2���,Definitions����,(7)】:
‘companion diagnostic’ means a device which is essential for the safe and effective use of a corresponding medicinal product to:
“伴隨診斷產(chǎn)品”指的是對(duì)于相應(yīng)藥品的安全和有效使用必不可少的器械�,用以:
(a) identify, before and/or during treatment, patients who are most likely to benefit from the corresponding medicinal product;
在治療之前和/或治療期間�,識(shí)別最有可能受益于相應(yīng)藥品的患者;
or 或者
(b) identify, before and/or during treatment, patients likely to be at increased risk of serious adverse reactions as a result of treatment with the corresponding medicinal product;
在治療之前和/或治療期間����,識(shí)別可能由于使用相應(yīng)藥品治療而導(dǎo)致嚴(yán)重不良反應(yīng)風(fēng)險(xiǎn)增加的患者;
在IVDR法規(guī)立法背景第(11)條指出:
Companion diagnostics are essential for defining patients' eligibility for specific treatment with a medicinal product through the quantitative or qualitative determination of specific markers identifying subjects at a higher risk of developing an adverse reaction
to the medicinal product in question or identifying patients in the population for whom the therapeutic product has been adequately studied, and found safe and effective. Such biomarker
or biomarkers can be present in healthy subjects and/or in patients.
伴隨診斷產(chǎn)品對(duì)于通過(guò)定量或定性確定特定標(biāo)志物來(lái)確定患者是否有資格接受藥物的特定治療至關(guān)重要����,該標(biāo)志物用于識(shí)別對(duì)所述藥物產(chǎn)生不良反應(yīng)的風(fēng)險(xiǎn)較高的受試者���,或用于識(shí)別人群中治療產(chǎn)品已被充分研究并發(fā)現(xiàn)安全有效的患者����。這樣的一種或多種生物標(biāo)志物可以存在于健康受試者和/或患者中。
立法背景第(12)條指出:
(12) Devices that are used with a view to monitoring treatment with a medicinal product in order to ensure that the concentration of relevant substances in the human body is within the therapeutic window are not considered to be companion diagnostics.
用于監(jiān)測(cè)藥物治療以確保人體內(nèi)相關(guān)物質(zhì)濃度在治療窗口內(nèi)的器械���,不被視為伴隨診斷產(chǎn)品。
但是在IVDR法規(guī)中�,并沒(méi)有找到伴隨診斷產(chǎn)品的相關(guān)舉例。
FDA體外伴隨診斷產(chǎn)品指南中�,對(duì)體外伴隨診斷產(chǎn)品的定義如下:
An IVD companion diagnostic device could be essential for the safe and effective use of a corresponding therapeutic product to:
? Identify patients who are most likely to benefit from the therapeutic product
? Identify patients likely to be at increased risk for serious adverse reactions as a result of treatment with the therapeutic product
? Monitor response to treatment with the therapeutic product for the purpose of adjusting treatment (e.g., schedule, dose, discontinuation) to achieve improved safety or effectiveness
? Identify patients in the population for whom the therapeutic product has been adequately studied, and found safe and effective, i.e., there is insufficient information about the safety and effectiveness of the therapeutic product in any other population
(這一條雖不被IVDR法規(guī)中的伴隨診斷產(chǎn)品定義所覆蓋�,但是與IVDR法規(guī)立法背景中第(11)條的描述一致)
與IVDR法規(guī)中不同的是����,F(xiàn)DA將“監(jiān)測(cè)對(duì)治療產(chǎn)品治療的反應(yīng)�,以調(diào)整治療(如計(jì)劃����、劑量、停藥)�,從而提高安全性或有效性”的器械也作為體外伴隨診斷產(chǎn)品����。看上去與IVDR立法背景第(12)條的要求相悖�。
那么我們來(lái)看一下FDA體外伴隨診斷產(chǎn)品指南中關(guān)于伴隨診斷產(chǎn)品的一個(gè)示例:
HER-2測(cè)試,用于確定患者是否可以接受赫賽?。ㄇ字閱慰梗┲委煟撍幬镞m用于治療轉(zhuǎn)移性乳腺癌和胃癌���。赫賽汀在HER-2標(biāo)志物陰性人群中缺乏有效性���,也有可能引起嚴(yán)重的不良反應(yīng)。
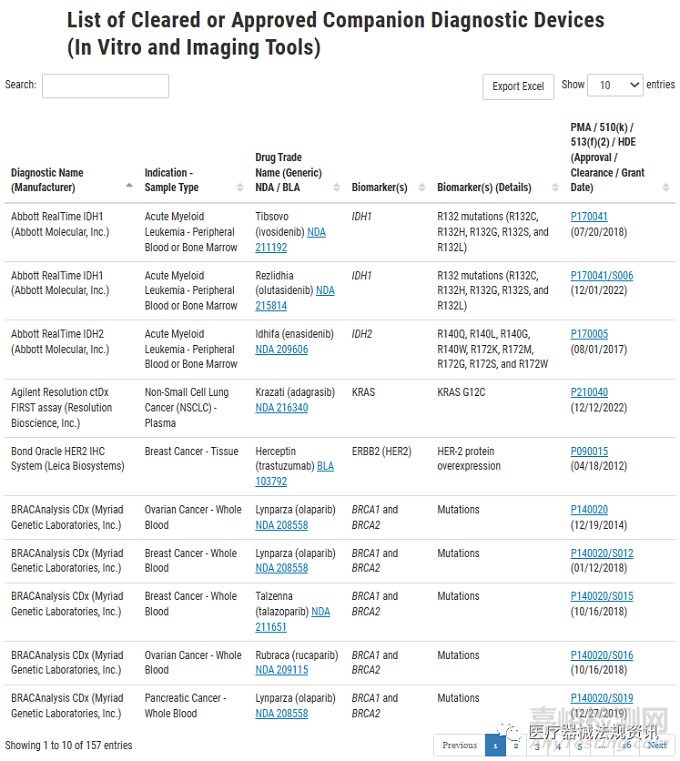
目前FDA cleared或批準(zhǔn)的伴隨診斷產(chǎn)品有157個(gè):
最近的一個(gè)是Abbott RealTime IDH1���,它是一種體外聚合酶鏈?zhǔn)椒磻?yīng)(PCR)檢測(cè)方法,用于定性檢測(cè)從人類(lèi)血液或骨髓提取的DNA中編碼五個(gè)IDH1 R132突變(R132C���、R132H����、R132G、R132S和R132L)的單核苷酸變體(SNV)����。Abbott RealTime IDH1被認(rèn)為有助于識(shí)別異檸檬酸脫氫酶-1(IDH1)突變的急性髓細(xì)胞白血?���。ˋML)患者���,用于TIBSOVO®(Ivosidenib)治療���。
部分截圖如下: