五��、Results summary
1) Provide an appropriate summary of data (in tabular and/or text format).including a summary from any analyses performed. For example: 提供適當(dāng)?shù)臄?shù)據(jù)摘要(以表格和/或文本格式)��,包括對(duì)所執(zhí)行的任何分析的摘要�����。例如:
? For quantitative assessments, provide appropriate summary parameters such as: the mean, standard deviation, minimum, and maximum for normal data; or summary parameters per the data analysis plan. 對(duì)于定量評(píng)估��,提供適當(dāng)?shù)膮R總參數(shù),例如:正常數(shù)據(jù)的平均值����、標(biāo)準(zhǔn)偏差、最小值和最大值�����;或每個(gè)數(shù)據(jù)分析計(jì)劃的匯總參數(shù)�����。
? For attribute or qualitative data, provide an appropriate summary of the number of observed characteristics by category (e.g., characteristic present/total observations, characteristic absent/total observations). Include statistical information such as confidence/reliability level, if applicable. 對(duì)于屬性或定性數(shù)據(jù)�����,按類別提供對(duì)觀察到的特征數(shù)量的適當(dāng)總結(jié)(例如�����,特征存在/總觀察��,特征缺失/總觀察)����。如果適用��,包括統(tǒng)計(jì)信息,例如置信度/可靠性水平��。
2) Specify whether the acceptance criteria (if applicable) were met.說(shuō)明是否滿足驗(yàn)收標(biāo)準(zhǔn)(如果適用)����。
3) Provide a brief explanation of study results that do not meet acceptance criteria, and/or protocol deviations that may have impacted the study results, conclusions, or data integrity, and describe how the resulting concerns were resolved. 簡(jiǎn)要解釋不符合驗(yàn)收標(biāo)準(zhǔn)的研究結(jié)果,和/或可能影響研究結(jié)果��、結(jié)論或數(shù)據(jù)完整性的方案偏差����,并描述如何解決由此產(chǎn)生的問(wèn)題。
測(cè)試結(jié)果:數(shù)據(jù)����、數(shù)據(jù)分析和方案偏差的描述(PS:其中統(tǒng)計(jì)信息,例如置信度/可靠性水平提問(wèn)較多)����。
六、Discussion/Conclusions
Your test report summaries should provide a discussion of the conclusions drawn from the test results. This section of the test report summary can be used to provide additional information regarding the testing conducted and/or observed test results(e.g., justification for the methods used to perform the testing, clinical/scientific engineering basis for the acceptance criteria, outlying and anomalous results). Note that a justification for methods and/or acceptance criteria is generally not needed if they were directly obtained from a FDA-recognized consensus standard or guidance document. A discussion of the test methods or acceptance criteria, if needed, can include how the testing relates to the use of the device in clinical practice or as described in literature. For example, tracking tests typically use a fixture that simulates the target anatomy. A discussion or justification for the tracking test fixture used would address how the testing relates to expected worst-case clinical use for the indicated anatomical location. For non-clinical bench performance tests that are conducted for characterization purposes, the conclusions should address the relationship between the results and the intended performance of the device. As noted above, a brief discussion of how the test results support the overall submission can be included in the test report summary or another location in your submission.
討論和結(jié)論:提供一個(gè)從測(cè)試結(jié)果得出的結(jié)論的討論
測(cè)試報(bào)告摘要的這一部分可用于提供有關(guān)進(jìn)行的測(cè)試和/或觀察到的測(cè)試結(jié)果的附加信息(例如����,用于執(zhí)行測(cè)試的方法的合理性、驗(yàn)收標(biāo)準(zhǔn)的臨床/科學(xué)工程基礎(chǔ)、異常和異常 結(jié)果)�����。請(qǐng)注意��,如果方法和/或驗(yàn)收標(biāo)準(zhǔn)是直接從 FDA 認(rèn)可的共識(shí)標(biāo)準(zhǔn)或指導(dǎo)文件中直接獲得的�����,則通常不需要對(duì)其進(jìn)行論證��。如果需要����,對(duì)測(cè)試方法或驗(yàn)收標(biāo)準(zhǔn)的討論可以包括測(cè)試如何與臨床實(shí)踐中或文獻(xiàn)中描述的設(shè)備使用相關(guān)。例如����,跟蹤測(cè)試通常使用模擬目標(biāo)解剖結(jié)構(gòu)的夾具�����。對(duì)所用跟蹤測(cè)試夾具的討論或論證將解決測(cè)試如何與指定解剖位置的預(yù)期最壞情況臨床使用相關(guān)��。對(duì)于為表征目的而進(jìn)行的非臨床工作臺(tái)性能測(cè)試,結(jié)論應(yīng)說(shuō)明結(jié)果與設(shè)備預(yù)期性能之間的關(guān)系��。如上所述��,關(guān)于測(cè)試結(jié)果如何支持整體提交的簡(jiǎn)短討論可以包含在測(cè)試報(bào)告摘要或您提交的其他位置�����。
七����、Location of complete test report
We recommend that you identify the location (e.g., appendix and/orpage number) for each complete test report for which a summary isprovided,if applicable.
我們建議您確定每個(gè)完整的測(cè)試報(bào)告的位置(例如,附錄和/或頁(yè)碼)�����,并為其提供摘要(如果適用)��。
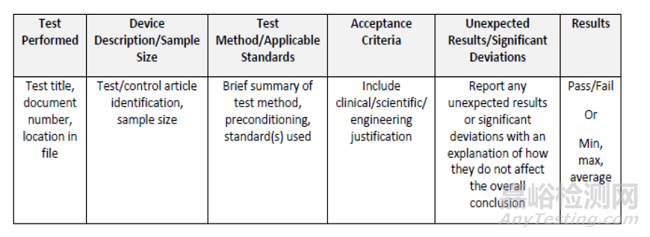
八����、Summary table (optional)
As an alternative to a written narrative for each non-clinical benchperformance test, a tabulated summary can be provided to organizethe information recommended in a test report summary (see belowfor example). lf a summary table is used, it is still recommendedthat a narrative discussion of the results/conclusions be provided asdescribed above in Section IL.A.6, when needed.匯總表 (可選)
作為每個(gè)非臨床工作臺(tái)性能測(cè)試的書面敘述的替代方案,可以提供表格摘要來(lái)組織測(cè)試報(bào)告摘要中推薦的信息 (參見(jiàn)下面的示例)��。如果使用匯總表�����,仍建議提供對(duì)結(jié)果/結(jié)論的敘述性討論。