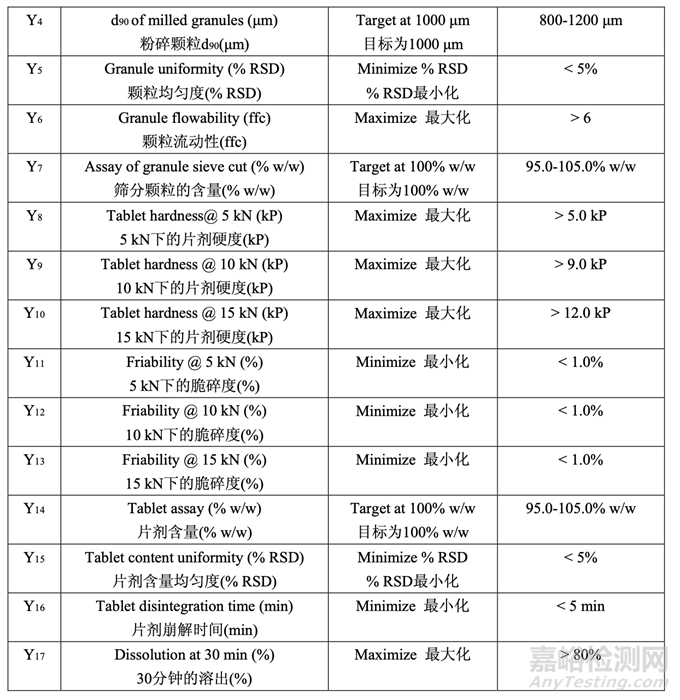
This is an example pharmaceutical development report illustrating how ANDA applicants can move toward implementation of Quality by Design (QbD). The purpose of the example is to illustrate the types of pharmaceutical development studies ANDA applicants may use as they implement QbD in their generic product development and to promote discussion on how OGD would use this information in review.
FDA官網(wǎng)中一個(gè)有關(guān)藥物開(kāi)發(fā)報(bào)告的實(shí)例,用以說(shuō)明申請(qǐng)人如何實(shí)施質(zhì)量源于設(shè)計(jì)(QbD)�����。 該實(shí)例的目的是說(shuō)明ANDA申請(qǐng)人在其仿制藥開(kāi)發(fā)過(guò)程中實(shí)施QbD時(shí)�����,可使用的藥物開(kāi)發(fā)研究的類型�����,同時(shí)促進(jìn)探討OGD在審評(píng)中如何使用該信息。
本文主要概述了制劑生產(chǎn)工藝開(kāi)發(fā)的風(fēng)險(xiǎn)評(píng)估要點(diǎn)�����,以及預(yù)碾壓混合�����、潤(rùn)滑�����、碾壓和粉碎�����。
2.3 Manufacturing Process Development 生產(chǎn)工藝的開(kāi)發(fā)
Note to Reader: There are various approaches to process development used in the generic pharmaceutical industry. This is one of many possible examples. All QbD approaches to process development should identify the critical material attributes (CMAs) and critical process parameters (CPPs) for each process step. A firm may choose to do this through reference to documented prior knowledge or through empirical experiments on a range of process scales building toward the exhibit scale and proposed commercial scale. The process development of pre-roller compaction blending and lubrication is an example of experimentally determining CPPs when there is variation in an input material attribute. QbD emphasizes building understanding to avert failures during scale-up. The multivariate experiments described here are a step toward defining acceptable ranges for CPPs and CMAs.
仿制藥行業(yè)中使用的工藝開(kāi)發(fā)有多種方法�����。這是多種可能實(shí)例中的一種�����。工藝開(kāi)發(fā) 的所有QbD方法應(yīng)確定每個(gè)工藝步驟的關(guān)鍵物料屬性(CMAs)和關(guān)鍵工藝參數(shù)(CPPs)。公司 可選擇通過(guò)參考記錄的先前知識(shí)或通過(guò)工藝規(guī)模范圍內(nèi)建立申報(bào)規(guī)模和擬定工業(yè)規(guī)模的實(shí) 證實(shí)驗(yàn)來(lái)進(jìn)行�����。預(yù)碾壓混合和潤(rùn)滑工藝的開(kāi)發(fā)是當(dāng)輸入物料屬性發(fā)生變化時(shí)�����,通過(guò)實(shí)驗(yàn)確定 CPPs的例子�����。QbD強(qiáng)調(diào)放大期間應(yīng)建立理解以避免失敗�����。此處描述的多元實(shí)驗(yàn)是邁向定義CPPs和CMAs可接受范圍的一步�����。
Steps to establish process understanding are as follows:
建立工藝?yán)斫獾牟襟E如下:
Identify all possible known material attributes and process parameters that could impact the performance of the process.
確定所有可能可影響工藝性能的已知物質(zhì)屬性和工藝參數(shù)�����。
Use risk assessment and scientific knowledge to identify potentially high risk attributes and/or parameters.
使用風(fēng)險(xiǎn)評(píng)估和科學(xué)知識(shí)以確定潛在的高風(fēng)險(xiǎn)屬性和/或參數(shù)。
Identify levels or ranges of these potentially high risk attributes and/or parameters. 確定這些潛在的高風(fēng)險(xiǎn)屬性和/或參數(shù)的水平或范圍。
Design and conduct experiments, using DOE when appropriate. 如適用�����,使用DOE進(jìn)行設(shè)計(jì)并進(jìn)行實(shí)驗(yàn)�����。
Analyze the experimental data to determine if a material attribute or process parameter is critical.
分析實(shí)驗(yàn)數(shù)據(jù)以確定物質(zhì)屬性或工藝參數(shù)是否關(guān)鍵。
- A material attribute or process parameter is critical when a realistic change in that attribute or parameter can significantly impact the quality of the output material.
關(guān)鍵的物質(zhì)屬性或工藝參數(shù)是當(dāng)屬性或參數(shù)實(shí)際變化時(shí),可顯著影響輸出物料質(zhì)量�����。 Develop a control strategy. 開(kāi)發(fā)控制策略
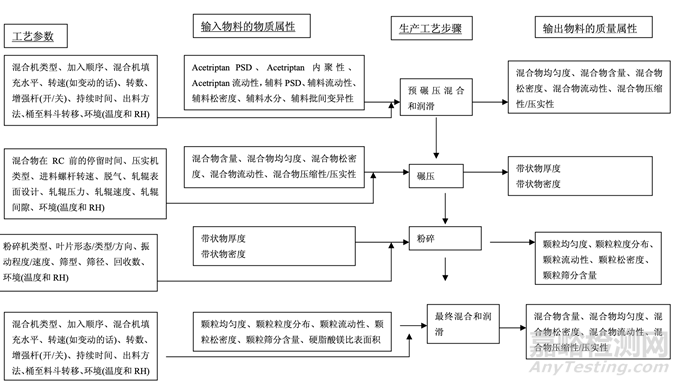
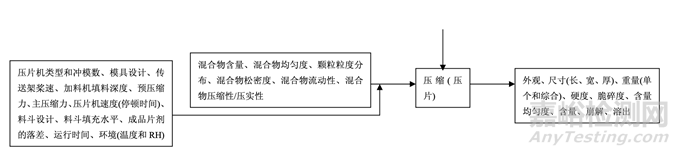
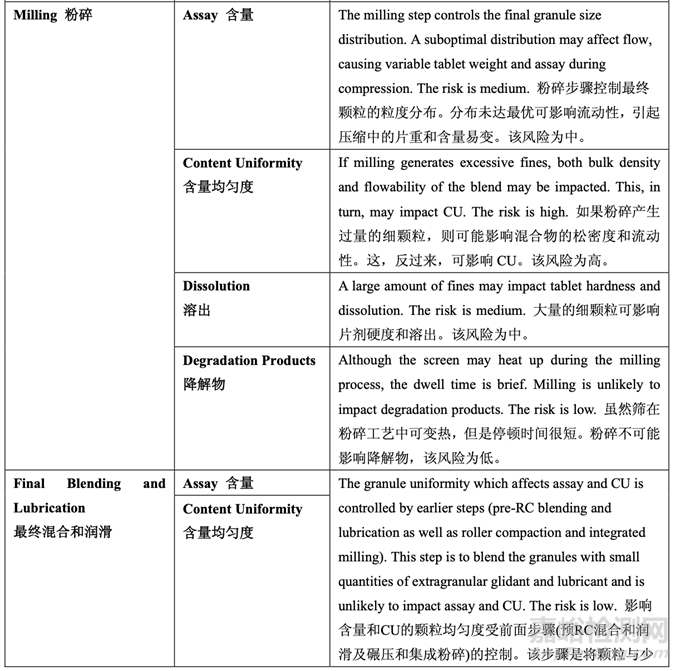
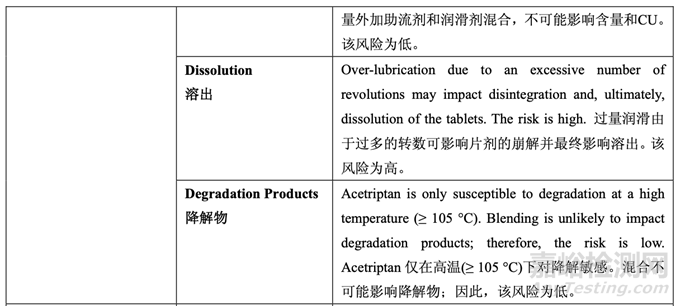
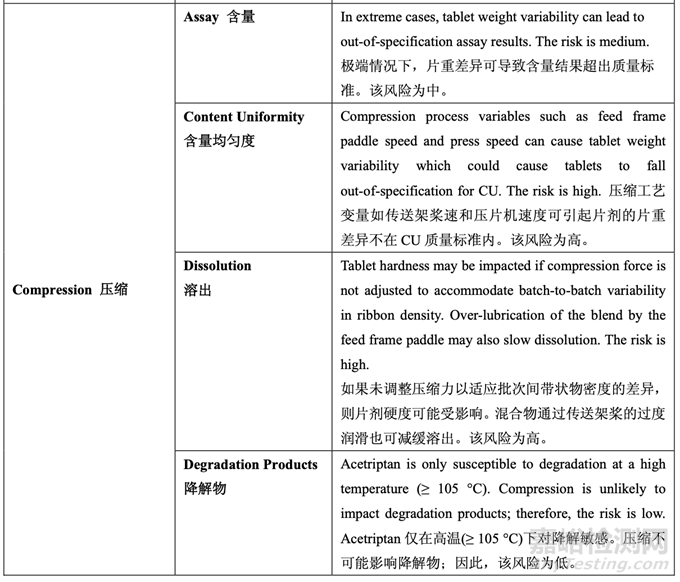
As discussed in Section 2.2.1.3 Process Selection, roller compaction was chosen as an appropriate granulation method to avoid drug product degradation and the equipment train was selected. Figure 21 presents the process map for the finalized formulation of Generic Acetriptan Tablets, 20 mg. Each process step in the manufacturing process is listed in the sequence of occurrence. It also presents the material attributes and process parameters that can potentially impact intermediate and finished product quality attributes. The material attributes of the input materials and the process parameters used at the very first process step determine the quality attributes of the output material (intermediate) produced at this step. Material attributes of the intermediate from this step and process parameters of the subsequent process step in the manufacturing process will determine quality attributes of the next intermediate and, eventually, those of the finished drug product. This cycle repeats until the final process step where finished drug product is manufactured and the product quality attributes are evaluated. This map was used to guide the risk assessments performed during process development.
如2.2.1.3節(jié)工藝選擇所述�����,選擇碾壓作為一種適宜制粒方法以避免制劑降解并選擇了設(shè)備培 訓(xùn)�����。圖21為仿制藥20 mg Acetriptan片最終確定的處方的工藝圖�����。生產(chǎn)工藝中的每個(gè)工藝步驟 按發(fā)生順序列出�����。也顯示了可潛在影響中間體和成品質(zhì)量屬性的物料屬性和工藝參數(shù)�����。第一 道工藝步驟使用的輸入物料的物質(zhì)屬性和工藝參數(shù)決定了該步驟生產(chǎn)的輸出物料(中間體) 的質(zhì)量屬性。該步驟中間體的物質(zhì)屬性和生產(chǎn)工藝中隨后工藝步驟的工藝參數(shù)將決定下一個(gè) 中間體的質(zhì)量屬性并最終決定成品制劑的質(zhì)量屬性�����。該循環(huán)重復(fù)直至生產(chǎn)成品制劑和評(píng)估產(chǎn) 品質(zhì)量屬性的最后的工藝步驟�����。該工藝圖用于指導(dǎo)工藝開(kāi)發(fā)中進(jìn)行的風(fēng)險(xiǎn)評(píng)估�����。 Manufacturing process development studies were conducted at the 5.0 kg lab scale, corresponding to 25,000 units.
在 5.0 kg 實(shí)驗(yàn)室規(guī)模,相當(dāng)于 25,000 單位下進(jìn)行生產(chǎn)工藝開(kāi)發(fā)研究�����。
Figure 21. Process map for Generic Acetriptan Tablets, 20 mg
圖 21 仿制藥 20 mg Acetriptan 片的工藝圖
2.3.1 Initial Risk Assessment of the Drug Product Manufacturing Process
藥品生產(chǎn)工藝的初始風(fēng)險(xiǎn)評(píng)估
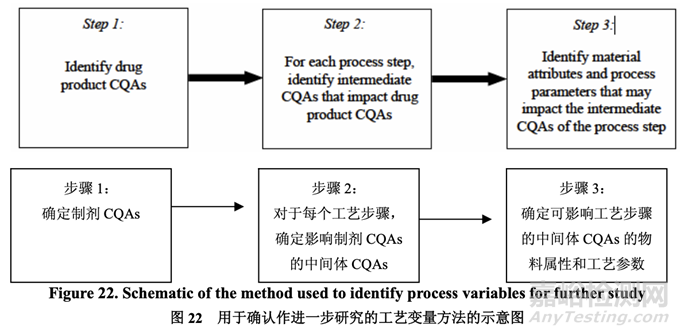
A risk assessment of the overall drug product manufacturing process was performed to identify the high risk steps that may affect the CQAs of the final drug product. Subsequently, the intermediate CQAs of the output material from each process step that impact the final drug product CQAs were identified. For each process step, a risk assessment was conducted to identify potentially high risk process variables which could impact the identified intermediate CQAs and, ultimately, the drug product CQAs. These variables were then investigated in order to better understand the manufacturing process and to develop a control strategy to reduce the risk of a failed batch. This method of identifying process variables for further study is illustrated in Figure 22 and is applied in each process step risk assessment.
進(jìn)行整體藥品生產(chǎn)工藝的風(fēng)險(xiǎn)評(píng)估以確定可能影響最終藥品 CQAs 的高風(fēng)險(xiǎn)步驟�����。隨后�����,確 定影響最終藥品 CQAs 的各個(gè)工藝步驟輸出物料的中間體 CQAs�����。對(duì)于各個(gè)工藝步驟�����,進(jìn)行 風(fēng)險(xiǎn)評(píng)估以確定可能影響已確定的中間體 CQAs 并最終影響藥品 CQAs 的潛在高風(fēng)險(xiǎn)工藝變 量。然后研究這些變量以便更好的理解生產(chǎn)工藝并開(kāi)發(fā)一種控制策略以降低失敗批的風(fēng)險(xiǎn)�����。 這種用于進(jìn)一步研究以確定工藝變量方法的說(shuō)明見(jiàn)圖 22 并用于各個(gè)工藝步驟風(fēng)險(xiǎn)評(píng)估�����。
The initial risk assessment of the overall manufacturing process is shown in Table 32 and justifications are provided in Table 33. Previous experience with these process steps was used to determine the degree of risk associated with each process step and its potential to impact the CQAs of the finished drug product.
整體生產(chǎn)工藝的初始風(fēng)險(xiǎn)評(píng)估見(jiàn)表 32�����,依據(jù)見(jiàn)表 33�����。使用這些工藝步驟的先前經(jīng)驗(yàn)用于決 定與各個(gè)工藝步驟相關(guān)的風(fēng)險(xiǎn)程度和其影響成品制劑 CQAs 的潛在性�����。
Further risk assessment was performed subsequently on each high risk process step to identify which process variables may potentially impact the intermediate CQAs. Evaluation of all possible process variables that could potentially impact the quality attributes of the output material of any given process step is not feasible; therefore, some of the variables were set constant based on current understanding.
隨后對(duì)各個(gè)高風(fēng)險(xiǎn)工藝進(jìn)行進(jìn)一步的風(fēng)險(xiǎn)評(píng)估以確定哪些工藝變量可潛在影響中間體 CQAs�����。評(píng)估所有可能的工藝變量�����,可潛在影響任何給定工藝步驟輸出物料的質(zhì)量屬性�����,是 不可行的;因此�����,基于現(xiàn)行的理解�����,設(shè)置某些變量為常數(shù)。
2.3.2 Pre-Roller Compaction Blending and Lubrication Process Development
預(yù)碾壓混合和潤(rùn)滑工藝開(kāi)發(fā)
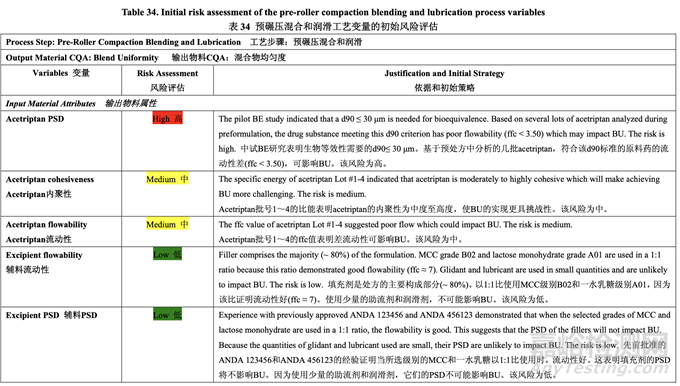
Initial Risk Assessment of the Pre-Roller Compaction Blending and Lubrication Process Variables
預(yù)碾壓混合和潤(rùn)滑工藝變量的初始風(fēng)險(xiǎn)評(píng)估
The initial risk assessment of the overall manufacturing process presented in Table 32 identified the risk of the pre-roller compaction blending and lubrication step to impact tablet content uniformity as high. Subsequently, blend uniformity was identified as an intermediate CQA of the powder blend from the pre-roller compaction blending and lubrication step. Process variables that could potentially impact blend uniformity were identified and their associated risk was evaluated. Table 34 presents the initial risk assessment for the pre-roller compaction blending and lubrication step.
表 32 所示的整體生產(chǎn)工藝的初始風(fēng)險(xiǎn)評(píng)估中�����,確定預(yù)碾壓混合和潤(rùn)滑為可影響片劑含量均 勻度的高風(fēng)險(xiǎn)步驟�����。隨后,混合物均勻度確定為預(yù)碾壓混合和潤(rùn)滑步驟的粉末混合物中間體 CQA。確定了可潛在影響混合物均勻度的工藝變量并評(píng)估了它們相關(guān)的風(fēng)險(xiǎn)�����。表 34 呈現(xiàn)了 預(yù)碾壓混合和潤(rùn)滑步驟的初始風(fēng)險(xiǎn)評(píng)估�����。
Effect of Acetriptan PSD and Number of Revolutions on Blend Uniformity
Acetriptan PSD
和轉(zhuǎn)數(shù)對(duì)混合物均勻度的影響
Due to its low solubility, acetriptan is milled to improve its bioavailability. The milled drug substance has poor flow characteristics and is cohesive. Thus, roller compaction is performed prior to compression to achieve tablet content uniformity. The success of roller compaction to produce uniform granules is largely contingent on the uniformity of the blend achieved during the preceding blending and lubrication step.
由于其溶解度低�����,粉碎acetriptan以提高其生物利用度。粉碎的原料藥的特點(diǎn)是流動(dòng)性差和具 有內(nèi)聚性。因此�����,在壓縮前進(jìn)行碾壓以達(dá)到片劑含量均勻度�����。成功碾壓生產(chǎn)均勻的顆粒主要 視之前混合和潤(rùn)滑步驟中達(dá)到的混合物的均勻度而定。
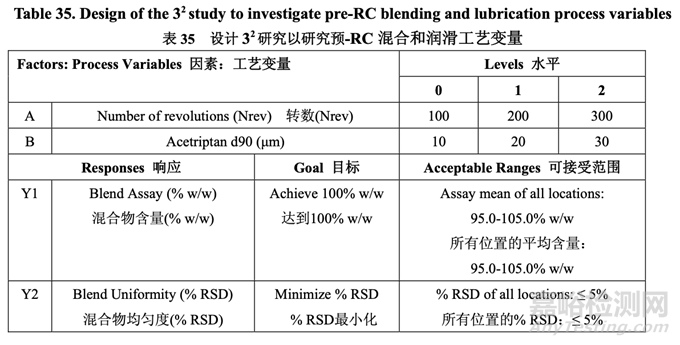
The pilot PK study suggested that Generic Acetriptan Tablets, 20 mg, with a drug substance d90 of 30 μm (d50 of 24 μm) or less would be bioequivalent to the RLD. During formulation development, a PSD with a d90 less than 14 μm led to flow and content uniformity issues. However, the blending process was fixed at that stage of development. Thus, it was important to determine if an optimized blending process could accommodate different acetriptan PSD without adversely impacting blend uniformity. A two-factor, three-level full factorial DOE, as shown in Table 35, was used to investigate the impact of acetriptan PSD (d90) and number of revolutions (Nrev) on blend uniformity. Blender fill level is also likely to impact blend uniformity based on the initial risk assessment, but this process parameter was evaluated subsequent to the DOE. The optimized formulation shown in Section 2.2.1.6 Table 29 was used for this study.
中試 PK 研究表明仿制藥 20 mg Acetriptan 片�����,其原料藥 d90 為 30 μm (d50 為 24 μm)或以下�����, 將生物等效于 RLD�����。在處方開(kāi)發(fā)中�����,d90 低于 14 μm 的 PSD 導(dǎo)致流動(dòng)性和含量均勻度問(wèn)題�����。 但是�����,在開(kāi)發(fā)的階段固定了混合工藝�����。因此�����,重要的是確定優(yōu)化的混合工藝是否能適應(yīng)不同 的 acetriptan PSD 而對(duì)混合物均勻度無(wú)不良影響�����。2 因素,3 水平全因子 DOE�����,如表 35 所示�����, 用于研究 acetriptan PSD (d90)和轉(zhuǎn)數(shù)(Nrev)對(duì)混合物均勻度的影響?����;旌蠙C(jī)填料水平也可能 影響混合物均勻度基于初始風(fēng)險(xiǎn)評(píng)估�����,但是該工藝參數(shù)在 DOE 之后進(jìn)行了評(píng)估�����。2.2.1.6 節(jié) 表 29 所示的優(yōu)化處方用于該研究。
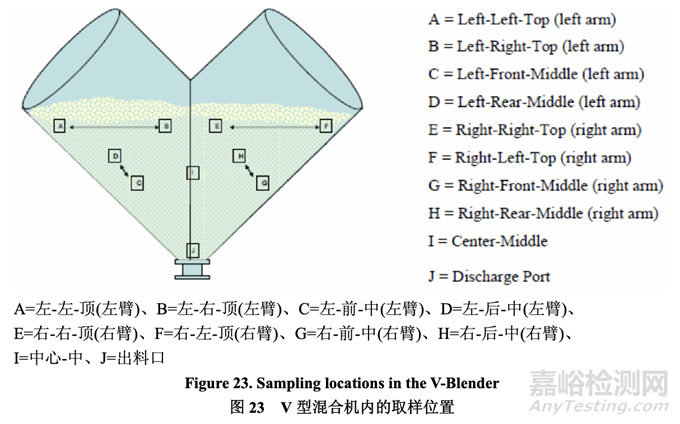
Each 5.0 kg batch was blended in a 16 qt blender operated at 20 rpm. To measure blend uniformity, sampling was performed at the 10 blender locations designated in Figure 23 at the end of the specified number of revolutions. The sample thief was calibrated such that the collected sample volume represented one to three unit doses of blend (200.0-600.0 mg).
20 rpm 下運(yùn)行的 16 qt 混合機(jī)內(nèi)混合各個(gè) 5.0 kg 批次�����。為測(cè)量混合物均勻度,在規(guī)定轉(zhuǎn)數(shù)的 末端�����,在圖 23 標(biāo)明的混合機(jī) 10 個(gè)位置進(jìn)行取樣�����。校正取樣器以便采集的樣本量代表混合物 的 1~3 個(gè)單位劑量(200.0~600.0 mg)�����。
The blend uniformity results are presented in Table 36.
混合物均勻度結(jié)果見(jiàn)表 36。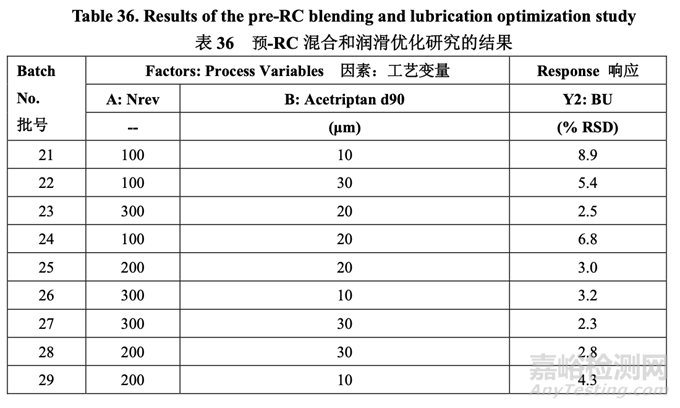
Based on the sum of squares of sequential models (i.e., linear, two factor interaction, quadratic and cubic), the highest order polynomial model was selected where the additional terms were significant and the model was not aliased. The model terms were further reduced based on the significance level (α = 0.05) using the backward model selection method.
基于順序模型(即線性,兩因素交互作用,二次和三次)的平方和�����,選擇最高階多項(xiàng)式模型�����,因?yàn)樵撃P椭懈郊禹?xiàng)顯著且模型不重疊�����?����;谑褂煤笙蚰P瓦x擇法的顯著水平(α = 0.05)�����,可 進(jìn)一步減少模型項(xiàng)�����。
Significant factors for blend uniformity 混合物均勻度的顯著因素
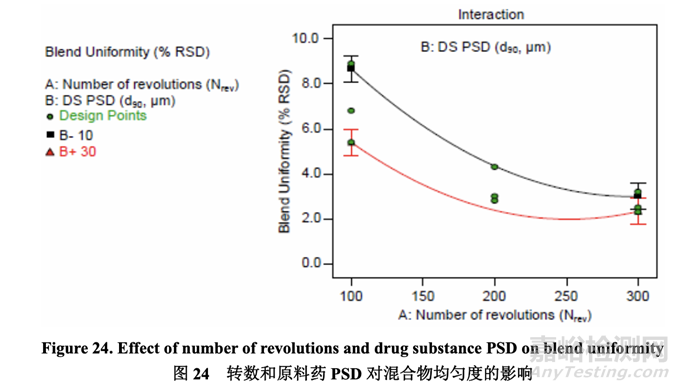
The effect of A (Nrev) and B (drug substance PSD) on blend uniformity was best described by a quadratic model where the significant factors were A, B, AB interaction and A2. The interaction plot below (Figure 24) shows that the blend uniformity response depended on the settings of the two factors. At a lower number of revolutions, the acetriptan PSD had a greater impact on blend uniformity than at a higher number of revolutions. At 100 revolutions, each of the three acetriptan PSD investigated failed to meet the predefined criterion of less than 5% RSD. 通過(guò)二次模型最好的描述了 A (Nrev)和 B (原料藥 PSD)對(duì)混合物均勻度的影響�����,其中顯著因 素為 A, B, AB 交互作用和 A2�����。交互作用圖以下(圖 24)顯示出混合物均勻度響應(yīng)依賴于兩個(gè) 因素的設(shè)置�����。更低轉(zhuǎn)數(shù)下的acetriptan PSD對(duì)混合物均勻度的影響較更高轉(zhuǎn)數(shù)大�����。100轉(zhuǎn)數(shù) 下�����,研究的 3 個(gè) acetriptan PSD 中的每一個(gè)都不符合預(yù)定義標(biāo)準(zhǔn):低于 5% RSD�����。
Significant factors for blend assay 混合物含量的顯著因素
Neither the number of revolutions nor the drug substance PSD had a significant impact on mean blend assay. Results were close to the target for each run and ranged from 98.7%-101.2% overall. 轉(zhuǎn)數(shù)和原料藥PSD都不顯著影響混合物平均含量�����。結(jié)果接近于每個(gè)批的目標(biāo)�����,總體范圍為 98.7%~101.2%�����。
Development of In-line NIR for Blending Endpoint Determination
開(kāi)發(fā)在線NIR用于混合終點(diǎn)判斷
Note to Reader: NIR method development and validation is beyond the scope of the pharmaceutical development report and the details are not discussed in this example. The validation report should be included in Section 3.2.P.5.3 Validation of Analytical Procedures. 致讀者:NIR方法的開(kāi)發(fā)和驗(yàn)證超出了藥物開(kāi)發(fā)報(bào)告的范圍�����,該實(shí)例中將不詳細(xì)討論�����。驗(yàn)證 報(bào)告應(yīng)包括在3.2.P .5.3節(jié)分析方法驗(yàn)證中�����。
In order to ensure a homogeneous blend for any input acetriptan drug substance d90 within the range of 10-30 μm, an in-line NIR spectrophotometric method was developed and validated. This technology allows a real-time response and can be used at the laboratory, pilot and commercial scale. During validation, blend uniformity data collected at various time points by the NIR method was compared to that obtained by traditional thief sampling followed by offline HPLC analysis and was found to be comparable.
為保證任何輸入的d90在10~30 μm范圍內(nèi)的acetriptan原料藥混合均勻,開(kāi)發(fā)并驗(yàn)證了一種在 線NIR分光光度法�����。該技術(shù)提供了實(shí)時(shí)響應(yīng)�����,可用于實(shí)驗(yàn)室�����,中試和工業(yè)規(guī)模�����。在驗(yàn)證中, 通過(guò)NIR法在各個(gè)時(shí)間點(diǎn)采集的混合物均勻度數(shù)據(jù)與通過(guò)傳統(tǒng)容器內(nèi)指定部位取樣再用離 線HPLC分析獲得的數(shù)據(jù)比較�����,發(fā)現(xiàn)兩者具有可比性�����。
Additionally, validation showed that blends deemed homogeneous by the NIR method ultimately produced tablets with acceptable content uniformity (% RSD < 5%). Based on these findings, the NIR method is capable of accurately assessing the real-time homogeneity of the blend and can be used to control the endpoint of the blending process. Further information regarding the NIR method development and validation can be found in Section 3.2.P.5.3 Validation of Analytical Procedures.
此外,驗(yàn)證表明通過(guò) NIR 法認(rèn)為均勻的混合物最終生產(chǎn)的片劑其含量均勻度合格(% RSD < 5%)�����。基于這些研究結(jié)果�����,NIR 法能準(zhǔn)確評(píng)估混合物的實(shí)時(shí)均勻性并可用于控制混合工藝的 終點(diǎn)�����。關(guān)于 NIR 法開(kāi)發(fā)和驗(yàn)證的另外信息見(jiàn) 3.2.P.5.3 節(jié)分析方法驗(yàn)證�����。
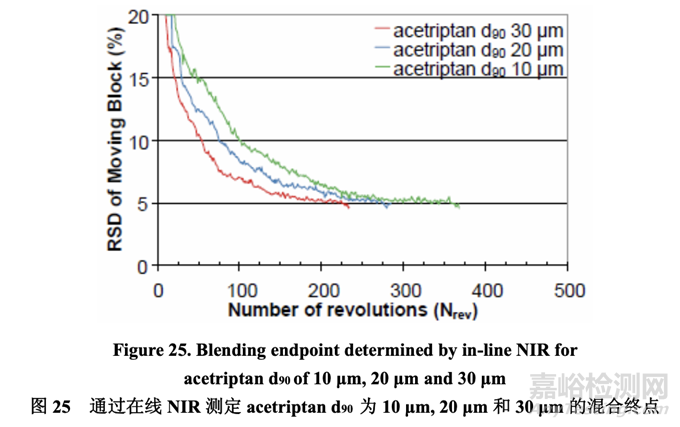
Three 5.0 kg batches (Batch Nos. 30-32) were manufactured using acetriptan with a d90 of 10 μm, 20 μm, and 30 μm, respectively. During blending, one spectrum was acquired non-invasively through the sight glass of the V-blender for each revolution as the V-blender was in the inverted position. The NIR spectra were preprocessed to minimize the effects of particle size and path length and to resolve the acetriptan peak. To assess the homogeneity of the blend, % RSD was calculated for each moving block of ten consecutive spectra and plotted as a function of number of revolutions. The blend was considered homogeneous once the % RSD was below 5% for ten consecutive measurements. This criterion ensured that brief excursions below the 5% threshold did not result in blending termination.
分別使用d90為10 μm, 20 μm和30 μm的acetriptan生產(chǎn)了各5.0 kg的3個(gè)批次(批號(hào)30~32)�����。在 混合中�����,通過(guò)V型混合機(jī)的觀察孔得到了每個(gè)轉(zhuǎn)速的無(wú)損光譜�����,當(dāng)V型混合機(jī)處于倒轉(zhuǎn)層位 時(shí)�����。預(yù)處理NIR光譜以使粒徑和光程長(zhǎng)度的影響最小化并分離acetriptan峰。為評(píng)估混合物的 均勻性,計(jì)算10個(gè)連續(xù)光譜中的每個(gè)移動(dòng)區(qū)的% RSD并根據(jù)轉(zhuǎn)數(shù)繪圖�����。一旦10個(gè)連續(xù)測(cè)量值 的% RSD低于5%�����,可認(rèn)為混合均勻。該標(biāo)準(zhǔn)保證了低于5%閾值的短漂移不發(fā)生混合終點(diǎn)�����。
For an acetriptan d90 of 10 μm, 20 μm and 30 μm, the blending endpoint determined by NIR as shown in Figure 25 was 368 revolutions, 285 revolutions and 234 revolutions, respectively. The blending uniformity showed rapid initial change through macro (convection) mixing followed by slower micro (diffusion) mixing.
對(duì)于 d90 為 10 μm, 20 μm 和 30 μm 的 acetriptan,通過(guò) NIR 判斷的混合終點(diǎn)分別為 368 轉(zhuǎn)數(shù)�����, 285 轉(zhuǎn)數(shù)和 234 轉(zhuǎn)數(shù)�����,如圖 25 所示�����?����;旌暇鶆蚨蕊@示先通過(guò)宏觀(對(duì)流)混合然后通過(guò)較慢的 微觀(擴(kuò)散)混合進(jìn)行快速初始變化。
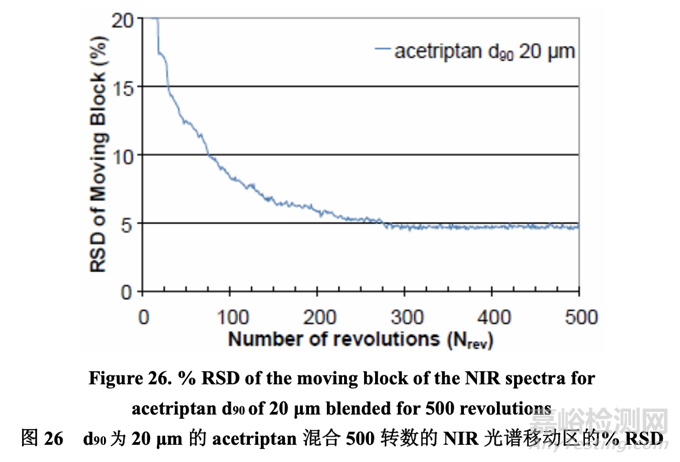
A fourth 5.0 kg batch (Batch No. 33) was manufactured using acetriptan with a d90 of 20 μm. The validated NIR method was used to determine the blending endpoint, but feedback control was not used to terminate the process. Blending was continued for a total of 500 revolutions to look for evidence of demixing. Figure 26 indicates that demixing did not occur as the % RSD did not increase when the batch was blended beyond the NIR-determined endpoint for a total of 500 revolutions.
使用d90為20μm的acetriptan生產(chǎn)了第4個(gè)5.0 kg的批次(批號(hào)33)�����。經(jīng)驗(yàn)證的NIR法用于 判斷混合終點(diǎn)�����,但是不使用反饋控制來(lái)終止過(guò)程。繼續(xù)混合至共 500 轉(zhuǎn)數(shù)以尋找混合物分離 的證據(jù)。圖26表明未發(fā)生混合物分離由于% RSD未增加�����,當(dāng)該批在NIR-判斷的終點(diǎn)范圍 外進(jìn)行混合至共 500 轉(zhuǎn)數(shù)時(shí)�����。
Effect of Blender Fill Level on Blend Uniformity
混合機(jī)填充水平對(duì)混合物均勻度的影響
Another study was performed to evaluate the impact of blender fill level on blend uniformity using acetriptan Lot #2 with a d90 of 20 μm. Each blend (Batch Nos. 34-36) was mixed in a 16 qt V-blender at 20 rpm and monitored using an NIR probe. Blend uniformity was achieved at approximately 280-290 revolutions for all three fill levels, 35%, 55% and 75%, indicating that blender fill level does not have a significant impact on the blending endpoint within the range of fill levels studied
使用d90為20μm的acetriptan批號(hào)2進(jìn)行了另一個(gè)研究以評(píng)估混合機(jī)填充水平對(duì)混合物均勻度 的影響�����。在16 qt V型混合機(jī)內(nèi),以20 rpm混合每個(gè)混合物(批號(hào)34~36)并用NIR探針監(jiān)測(cè)�����。 所有3個(gè)填充水平�����,35%, 55%和75%,在約280~290轉(zhuǎn)數(shù)時(shí)達(dá)到了混合均勻�����,表明混合機(jī)填 充水平在研究的填充水平范圍內(nèi)對(duì)混合終點(diǎn)無(wú)顯著影響。
Summary of Pre-Roller Compaction Blending and Lubrication Process Development
預(yù)碾壓混合和潤(rùn)滑工藝開(kāi)發(fā)的總結(jié)
Based on the results of the pre-roller compaction blending and lubrication studies, an in-line NIR method will be used to determine the blending endpoint. The number of revolutions needed to achieve blend uniformity differed depending on the acetriptan d90 in the range of 10-30 μm. Within the range of 35-75%, the blender fill level did not adversely impact blend uniformity. 基于預(yù)碾壓混合和潤(rùn)滑研究的結(jié)果,在線NIR法將用于判斷混合終點(diǎn)�����。需要達(dá)到混合均勻的 轉(zhuǎn)數(shù)是不同的,取決于d90在10~30 μm范圍內(nèi)的acetriptan�����。在35~75%范圍內(nèi)�����,混合機(jī)填充 水平對(duì)混合物均勻度無(wú)不良影響�����。
Updated Risk Assessment of the Pre-Roller Compaction Blending and Lubrication Process Variables
更新的預(yù)碾壓混合和潤(rùn)滑工藝變量的風(fēng)險(xiǎn)評(píng)估
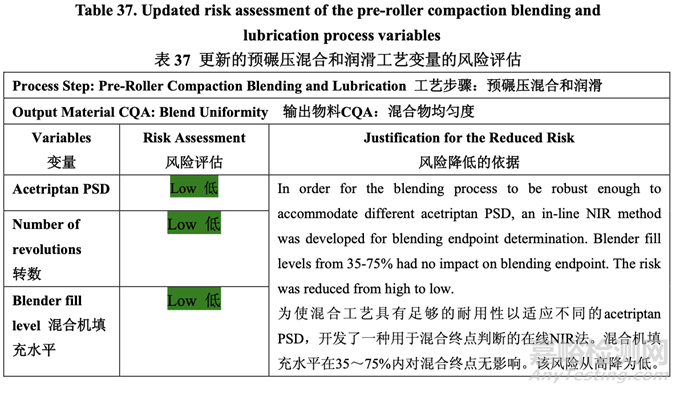
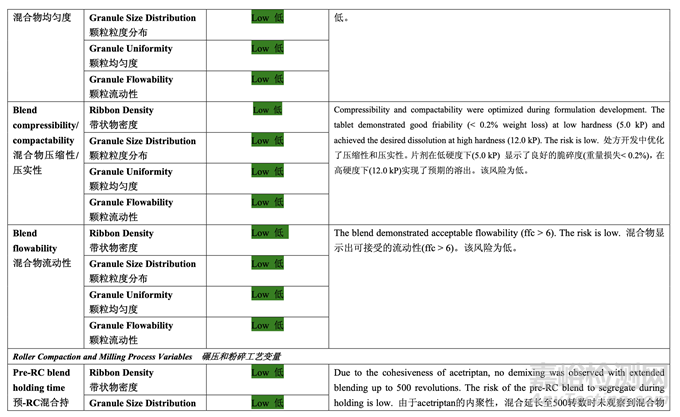
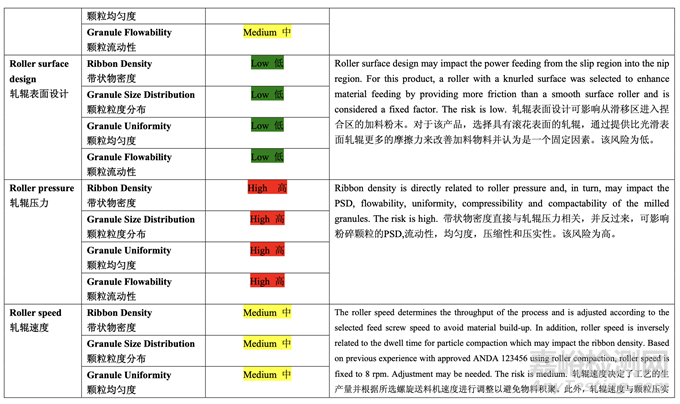
Table 37 presents the risk reduction for the pre-roller compaction blending and lubrication process as a result of the development studies. Only the process variables that were initially identified as high risk to the blend uniformity are shown.
表 37 呈現(xiàn)了由于開(kāi)發(fā)研究而降低了預(yù)碾壓混合和潤(rùn)滑工藝的風(fēng)險(xiǎn)�����。僅顯示了初始確定為混 合物均勻度高風(fēng)險(xiǎn)的工藝變量�����。
2.3.3 Roller Compaction and Integrated Milling Process Development
碾壓和集成粉碎工藝開(kāi)發(fā)
Initial Risk Assessment of the Roller Compaction and Integrated Milling Process Variables
碾壓和集成粉碎工藝變量的初始風(fēng)險(xiǎn)評(píng)估
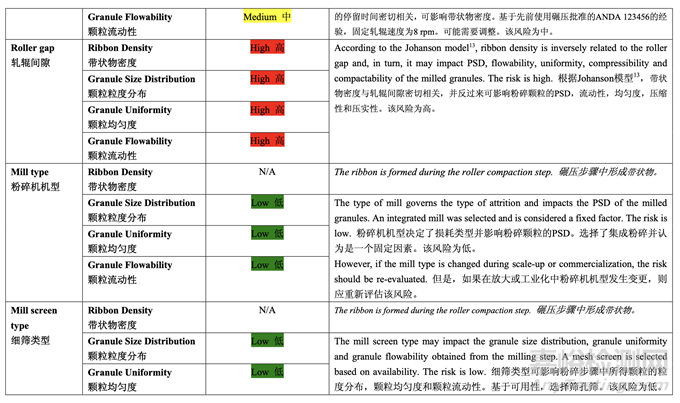
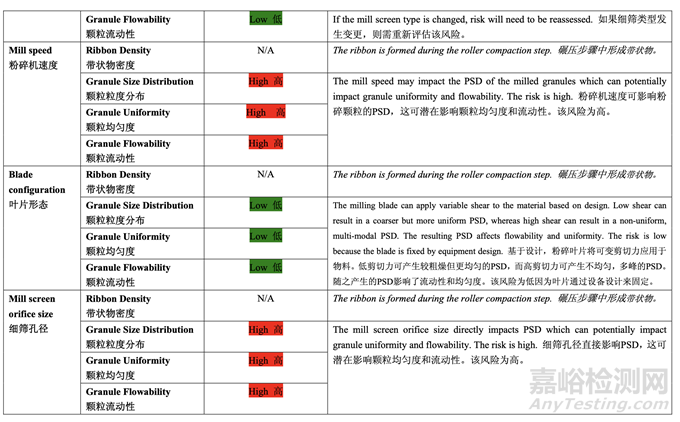
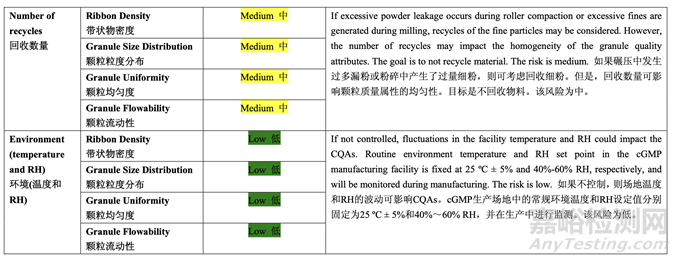
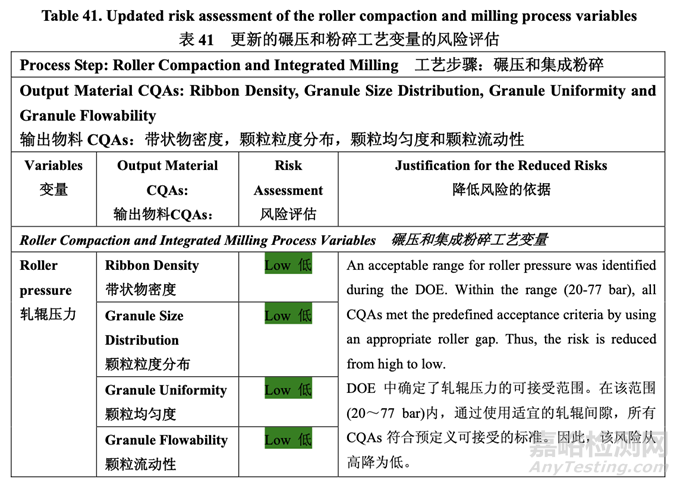
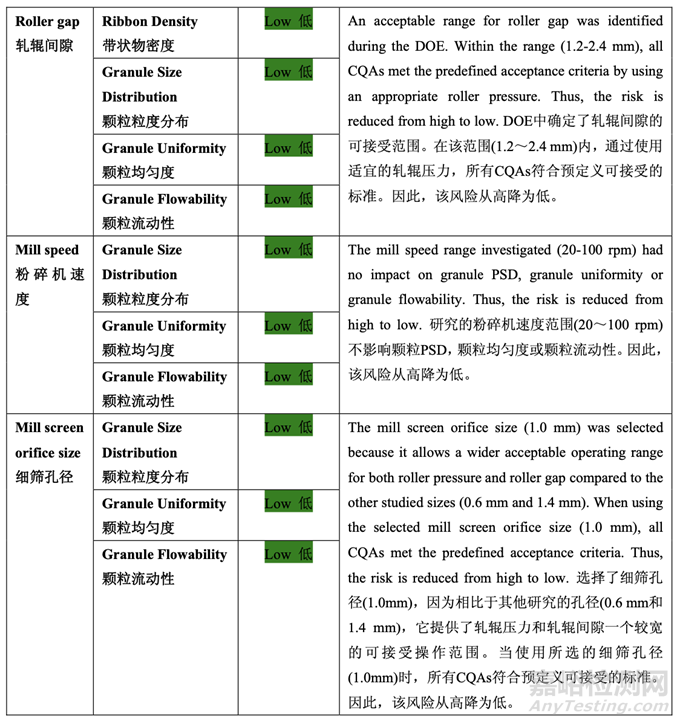
Based on the initial risk assessment of the overall manufacturing process shown in Table 32, the risk of the roller compaction step to impact tablet content uniformity and dissolution was identified as high and the risk of the milling step to impact tablet content uniformity was identified as high. Due to equipment availability, an Alexanderwerk10 WP120 roller compactor with integrated milling was used for this study. Therefore, these two steps were studied together. Subsequently, ribbon density, granule size distribution, granule uniformity and granule flowability were identified as the intermediate CQAs of the output material from the roller compaction and integrated milling step. Ribbon density is an intermediate CQA because it has a direct impact on granule particle size distribution, granule bulk and tapped density, granule flowability, and, ultimately, tablet hardness and dissolution. Granule size distribution, granule uniformity and granule flowability are intermediate CQAs because they are intimately related to tablet weight variability and content uniformity. The input material attributes and process parameters for this step that could potentially impact the four intermediate CQAs of the output material were identified and their associated risk was evaluated. The result of the initial risk assessment is summarized in Table 38.
基于整體生產(chǎn)工藝的初始風(fēng)險(xiǎn)評(píng)估�����,如表 32 所示,確定碾壓步驟是影響片劑含量均勻度和 溶出的高風(fēng)險(xiǎn),粉碎步驟是影響片劑含量均勻度的高風(fēng)險(xiǎn)�����。由于設(shè)備的可用性�����,該研究使用 帶集成粉碎的Alexanderwerk10 WP120壓實(shí)機(jī)。因此�����,這兩個(gè)步驟一起進(jìn)行了研究。隨后�����, 確定了帶狀物密度,顆粒粒度分布,顆粒均勻度和顆粒流動(dòng)性為碾壓和集成粉碎步驟的輸出 物料的中間體 CQAs�����。帶狀物密度為中間體 CQA�����,因?yàn)樗苯佑绊戭w粒粒度分布�����,顆粒松 密度和振實(shí)密度,顆粒流動(dòng)性并最終影響片劑硬度和溶出�����。顆粒粒度分布�����,顆粒均勻度和顆 粒流動(dòng)性為中間體 CQAs�����,因?yàn)樗鼈兣c片重差異和含量均勻度密切相關(guān)�����。確定了該步驟可潛 在影響輸出物料的 4 個(gè)中間體 CQAs 的輸入物料屬性和工藝參數(shù)并評(píng)估了它們相關(guān)的風(fēng)險(xiǎn)�����。 表 38 總結(jié)了初始風(fēng)險(xiǎn)評(píng)估的結(jié)果�����。
Effect of Roller Pressure, Roller Gap, Milling Speed and Mill Screen Orifice Size
軋輥壓力�����,軋輥間隙�����,粉碎速度和細(xì)篩孔徑的影響
The main objective of the study was to evaluate the effect of the roller compaction and integrated milling process parameters on the quality attributes of the ribbon, milled granules and finished drug product using DOE. The process parameters investigated were roller pressure, roller gap, milling speed and mill screen orifice size.
本研究的主要目的是使用DOE來(lái)評(píng)估碾壓和集成粉碎工藝參數(shù)對(duì)帶狀物,粉碎顆粒和成品制 劑質(zhì)量屬性的影響�����。研究的工藝參數(shù)是軋輥壓力�����,軋輥間隙,粉碎速度和細(xì)篩孔徑�����。
A preliminary feasibility experiment was conducted to study the effect of roller pressure on the quantity of by-pass material (un-compacted material). The study showed that within the roller pressure range of 20-80 bar, the quantity of by-pass material was less than 5% and the potency matched that of the blend fed into the roller compactor. Therefore, the roller pressure range of 20-80 bar was suitable for further studies. During the feasibility study, product temperature was monitored by a non-invasive measuring device. No significant increase (> 5°C) was observed. The ranges for roller gap, mill speed and mill screen orifice size were selected based on previous experience with approved ANDA 123456 and ANDA 456123.
進(jìn)行了一個(gè)初步可行性實(shí)驗(yàn)來(lái)研究軋輥壓力對(duì)旁通物料(未壓縮物料)數(shù)量的影響。研究表明 軋輥壓力在 20~80 bar 范圍內(nèi)�����,旁通物料數(shù)量低于 5%,效價(jià)與加料進(jìn)入壓實(shí)機(jī)的混合物匹 配�����。因此,20~80 bar 范圍內(nèi)的軋輥壓力適用于進(jìn)一步研究�����。在可行性研究中,產(chǎn)品溫度通 過(guò)無(wú)損測(cè)量設(shè)備監(jiān)測(cè)�����。觀察到無(wú)顯著性增加(> 5°C)�����。基于之前批準(zhǔn)的 ANDA 123456 和 ANDA 456123 的經(jīng)驗(yàn)�����,選擇了軋輥間隙�����,粉碎機(jī)速度和細(xì)篩孔徑的范圍�����。
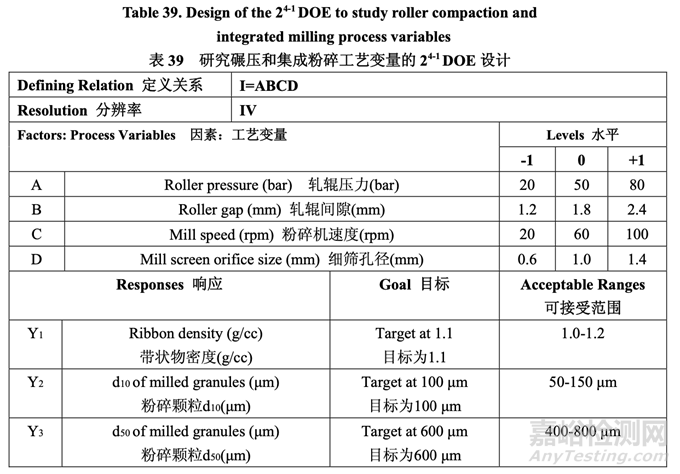
For this study, a 24-1 fractional factorial DOE was used and three center points were included to evaluate if any curvature effects exist. Table 39 presents the study design.
對(duì)于該研究�����,使用 24-1 部分析因 DOE 并包括了 3 個(gè)中心點(diǎn)以評(píng)估是否存在任何的曲率效應(yīng)。 研究設(shè)計(jì)見(jiàn)表 39�����。
Approximately 50.0 kg of the intragranular excipients and drug substance (Lot #2) were blended in a 150 L diffusive V-blender operated at 12 rpm. The blender was equipped with an NIR probe to monitor the blending endpoint (RSD < 5%, target revolutions ~234). The powder mixture was subdivided into 11 batches, each ~4.5 kg in size. The remaining 0.5 kg of powder was used as a control and was not roller compacted.
以12 rpm運(yùn)行的150 L擴(kuò)散型V型混合機(jī)內(nèi),將約50.0 kg內(nèi)加輔料和原料藥(批號(hào)2)進(jìn)行混合�����。 混合機(jī)裝備有NIR探針以監(jiān)測(cè)混合終點(diǎn)(RSD < 5%, 目標(biāo)轉(zhuǎn)數(shù)~234)。粉末混合物細(xì)分為11個(gè) 批次�����,每批數(shù)量各~4.5 kg�����。剩余0.5 kg粉末作為對(duì)照使用,不進(jìn)行碾壓�����。
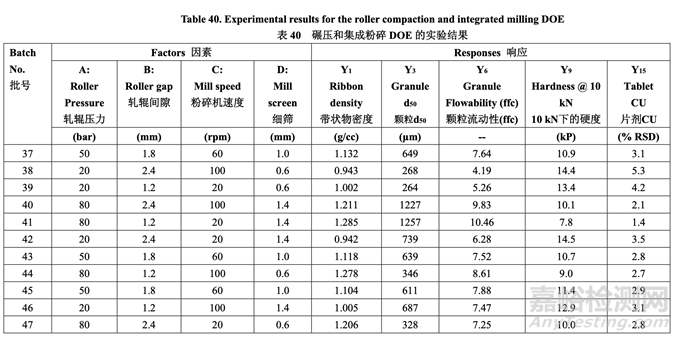
Each batch of blended powder was roller compacted using an Alexanderwerk WP120 (roller diameter 120 mm and roller width 25 mm) using the parameters defined in Table 40. The integrated milling unit on the Alexanderwerk WP120 is equipped with a ribbon crusher and a two-step milling apparatus. The ribbon is crushed into small flakes. The crushed flakes will first go through a coarse screen milling (sizing) step in which the rotor operates at 80% of the milling speed used for the second step. The second step is designed for final milling. In this study, the coarse screen size was fixed at 2.0 mm. The milling speed and milling screen orifice size of thesecond step are shown in Table 40.
每批混合的粉末使用Alexanderwerk WP120(軋輥直徑120 mm和軋輥寬度25 mm)并使用表40 定義的參數(shù)進(jìn)行碾壓�����。在Alexanderwerk WP120上的集成粉碎裝置裝備有帶條軋碎機(jī)和兩步 粉碎設(shè)備�����。將帶狀物粉碎為小片�����。粉碎的小片首先通過(guò)粗篩粉碎(分級(jí))步驟�����,葉輪以第二步 驟使用的80%粉碎速度運(yùn)行�����。設(shè)計(jì)第二步驟用于最終粉碎。該研究中�����,粗篩徑固定在2.0 mm�����。 第二步驟的粉碎速度和粉碎篩孔徑見(jiàn)表40
The milled granules were blended with talc for 100 revolutions in a 16 qt V-blender operated at 20 rpm. Magnesium stearate was then added and blended for an additional 80 revolutions. Each batch was compressed into tablets with a target weight of 200.0 mg. The tablet hardness and friability were studied as a function of main compression force. Three compression forces, 5 kN, 10 kN and 15 kN, were used. To study tablet assay, content uniformity (% RSD), disintegration and dissolution, the main compression force was adjusted to achieve a target hardness of 9.0 kP (8.0-10.0 kP was allowed).
以 20 rpm 運(yùn)行的 16 qt V 型混合機(jī)內(nèi),將粉碎顆粒與滑石粉混合 100 轉(zhuǎn)數(shù)�����。然后加入硬脂酸 鎂并混合另外 80 轉(zhuǎn)數(shù).每批壓制為目標(biāo)重量為 200.0 mg 的片劑。研究了片劑硬度和脆碎度與 主壓縮力的關(guān)系�����。使用了 3 種壓縮力�����,5 kN, 10 kN 和 15 kN�����。為研究片劑含量,含量均勻 度(% RSD)�����,崩解和溶出�����,調(diào)整主壓縮力以達(dá)到目標(biāo)硬度 9.0 kP(允許的范圍為 8.0~10.0 kP)�����。
Table 40 presents the experimental results for ribbon density, mean granule size (d50), granule flowability (ffc), tablet hardness observed at 10 kN force and tablet content uniformity (% RSD) (other responses not shown). 表40呈現(xiàn)了帶狀物密度,顆粒平均粒徑(d50)�����,顆粒流動(dòng)性(ffc),片劑在10 kN壓縮力下觀 察到的硬度和片劑含量均勻度(% RSD)的實(shí)驗(yàn)結(jié)果(其他響應(yīng)未顯示)�����。
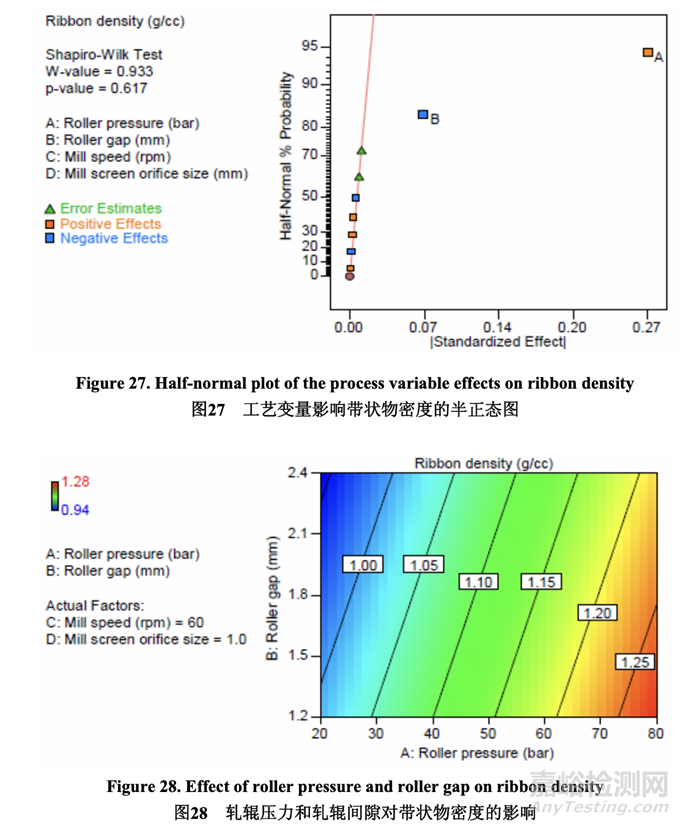
Significant factors for ribbon density 帶狀物密度的顯著因素
As shown in the half-normal plot (Figure 27), the significant factors affecting ribbon density were A (roller pressure) and B (roller gap). The effect of roller pressure and roller gap on ribbon density is presented in Figure 28. Ribbon density increased with increasing roller pressure (positive effect) and decreasing roller gap (negative effect).
如半正態(tài)圖(圖 27)所示,影響帶狀物密度的顯著因素為 A (軋輥壓力)和 B (軋輥間隙)�����。軋輥壓力和軋輥間隙對(duì)帶狀物密度的影響見(jiàn)圖 28�����。帶狀物密度隨 軋輥壓力(正作用)增加而增加�����,隨軋輥間隙(負(fù)作用)減少而增加。
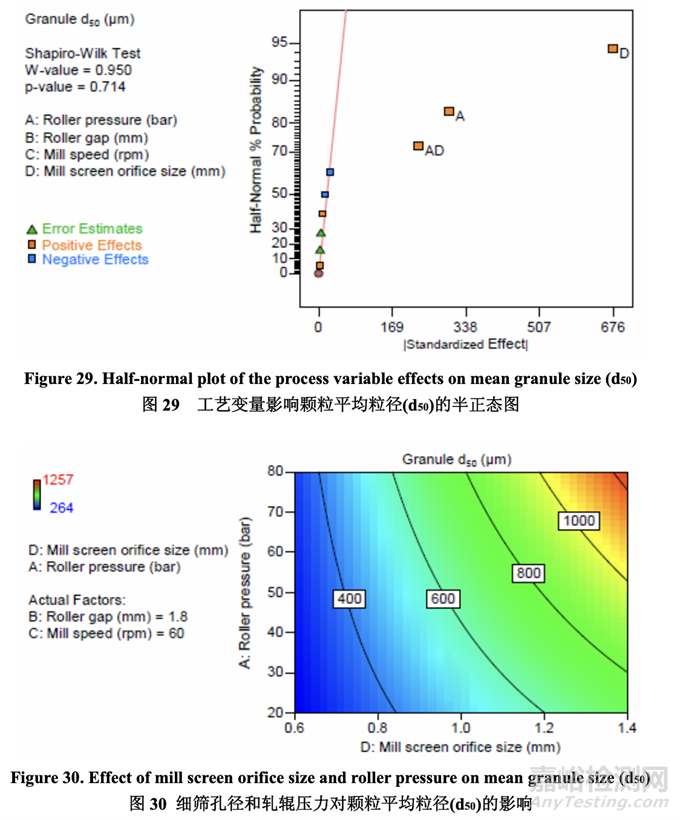
Significant factors for mean granule size (d50) 顆粒平均粒徑(d50)的顯著因素
The half-normal plot (Figure 29) shows that the significant factors affecting mean granule size (d50) were D (mill screen orifice size), A (roller pressure) and AD (their interaction). 半正態(tài)圖(圖29)表明�����,影響顆粒平均粒徑(d50)的顯著因素為D (細(xì)篩孔徑), A (軋輥壓力)和AD (它們的交互作用)。
The contour plot presented in Figure 30 shows the effect of mill screen orifice size and roller pressure on granule d50. It is evident that d50 increased with increasing mill screen orifice size and roller pressure (positive effect). These two parameters also exhibited a strong interaction (i.e., roller pressure showed a larger impact on mean granule size when using a larger mill screen orifice size).
圖 30 呈現(xiàn)的等高線圖顯示了細(xì)篩孔徑和軋輥壓力對(duì)顆粒 d50 的影響�����。很明顯�����,d50 隨細(xì)篩 孔徑和軋輥壓力(正作用)的增加而增加。這兩個(gè)參數(shù)也顯示了強(qiáng)烈的交互作用(即軋輥壓力顯示出對(duì)顆粒平均粒徑具有較大影響當(dāng)使用較大孔徑的細(xì)篩時(shí))�����。
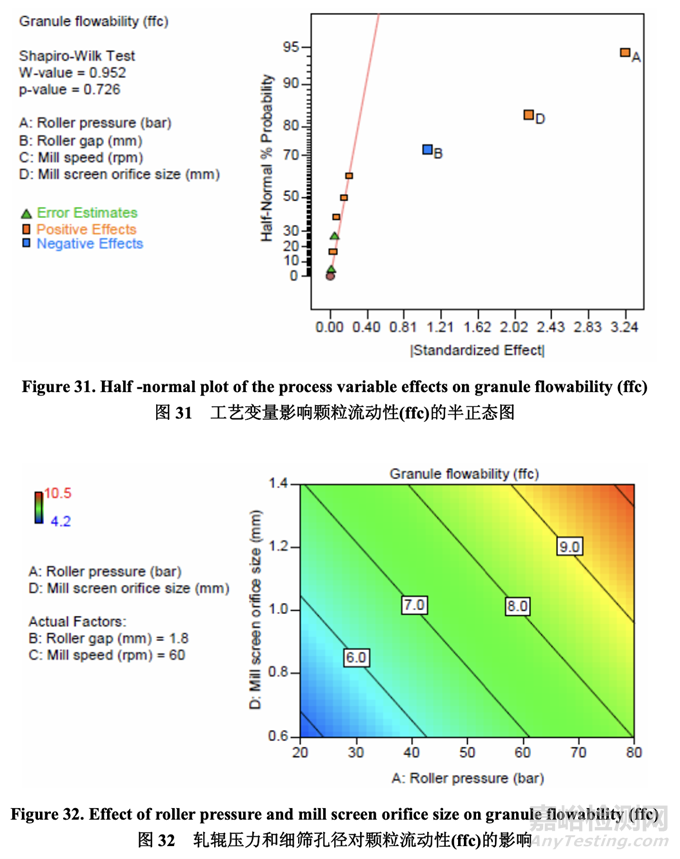
Significant factors for granule flowability 顆粒流動(dòng)性的顯著因素
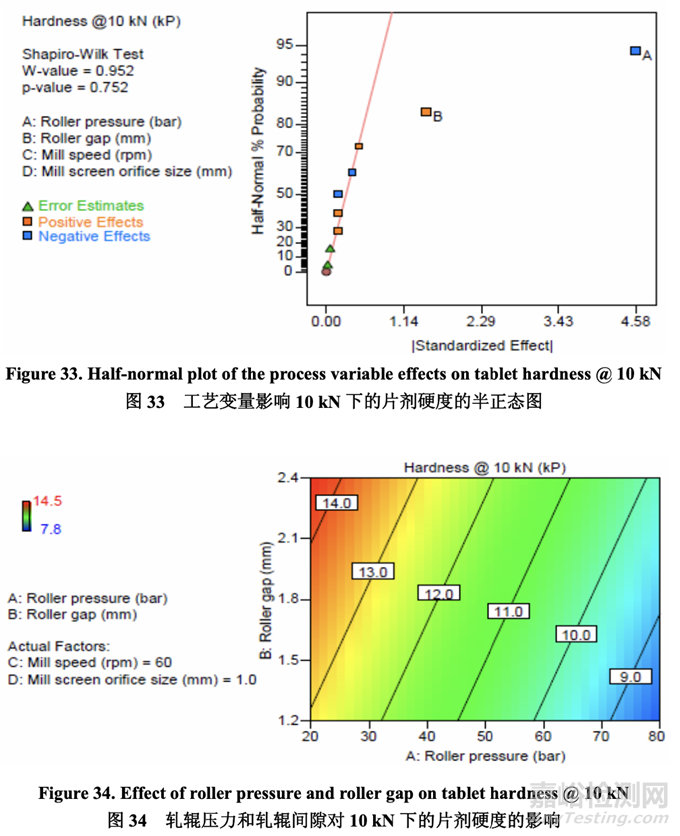
The flowability (represented by ffc value) of the granules after milling was determined using a ring shear tester. As shown in the half-normal plot (Figure 31), the significant factors affecting granule flowability were A (roller pressure), D (mill screen orifice size) and B (roller gap). The effect of roller pressure and mill screen orifice size on granule flowability is shown in Figure 32. Granule flowability improved with increasing roller pressure and mill screen orifice size. Roller gap also had an impact on granule flowability but to a lesser extent. 使用環(huán)剪儀測(cè)量粉碎后的顆粒流動(dòng)性(以 ffc 值表示)。如半正態(tài)圖(圖 31)所示�����,影響顆粒流 動(dòng)性的顯著因素為 A (軋輥壓力), D (細(xì)篩孔徑)和 B (軋輥間隙)�����。軋輥壓力和細(xì)篩孔徑對(duì)顆粒 流動(dòng)性的影響見(jiàn)圖 32。顆粒流動(dòng)性隨軋輥壓力和細(xì)篩孔徑增加而提高�����。軋輥間隙對(duì)顆粒流 動(dòng)性也有影響但程度較輕。
Significant factors for granule uniformity (% RSD) 顆粒均勻度(% RSD)的顯著因素
All batches demonstrated acceptable granule uniformity (ranging from 2.0-2.9% RSD) and none of the process variables showed a significant impact on this response.
所有批顯示出可接受的顆粒均勻度(RSD范圍為2.0~2.9%),無(wú)工藝變量顯示出對(duì)該響應(yīng)具有 顯著影響�����。
Significant factors for assay of granule sieve cuts 篩分顆粒含量的顯著因素
Approximately 10 g of granules were sampled from each batch and transferred to the top of a set of seven sieves stacked by decreasing size: 840 μm, 420 μm, 250 μm, 180 μm, 149 μm, 75 μm and pan (no opening for fine collection). The sieves were shaken for five minutes on a laboratory particle size analyzer. The assay of sieve cuts collected from each batch was analyzed. All batches demonstrated acceptable assay for each granule sieve cut (ranging from 98.2-102.0%). This data confirmed that segregation of the pre-roller compacted blend did not occur. None of the factors were shown to have a significant impact on the assay of granule sieve cuts.
每批取樣約10 g顆粒,轉(zhuǎn)移至一套按孔徑降序:840 μm, 420 μm, 250 μm, 180 μm, 149 μm, 75 μm和平地器皿(沒(méi)有用于細(xì)粉收集的孔)堆積的7個(gè)篩的頂部�����。在實(shí)驗(yàn)室粒度分析儀上振搖篩5 分鐘�����。分析每批采集的篩分含量。所有批顯示每批篩分顆粒的含量是合格的(范圍為98.2~ 102.0%)�����。該數(shù)據(jù)確認(rèn)預(yù)碾壓混合不發(fā)生分離�����。無(wú)因素顯示對(duì)篩分顆粒的含量有顯著影響。 Significant factors for tablet hardness 片劑硬度的顯著因素
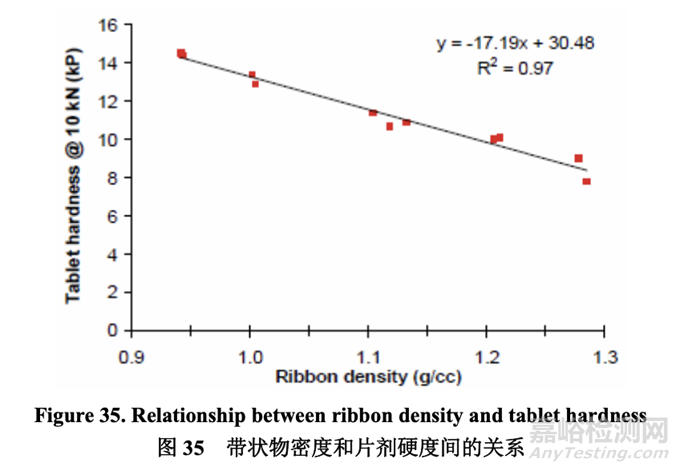
As shown in the half-normal plot (Figure 33), the significant factors affecting tablet hardness when compressed using 10 kN of force were A (roller pressure) and B (roller gap). The effect of roller pressure and roller gap on tablet hardness is presented in Figure 34. Tablet hardness decreased with increasing roller pressure and decreasing roller gap.
如半正態(tài)圖(圖 33)所示�����,當(dāng)使用 10 kN 力壓縮時(shí)�����,影響片劑硬度的顯著因素為 A (軋輥壓力) 和 B (軋輥間隙)。軋輥壓力和軋輥間隙對(duì)片劑硬度的影響見(jiàn)圖 34�����。片劑硬度隨隨軋輥壓力 增加而減少,隨軋輥間隙減少而減少�����。
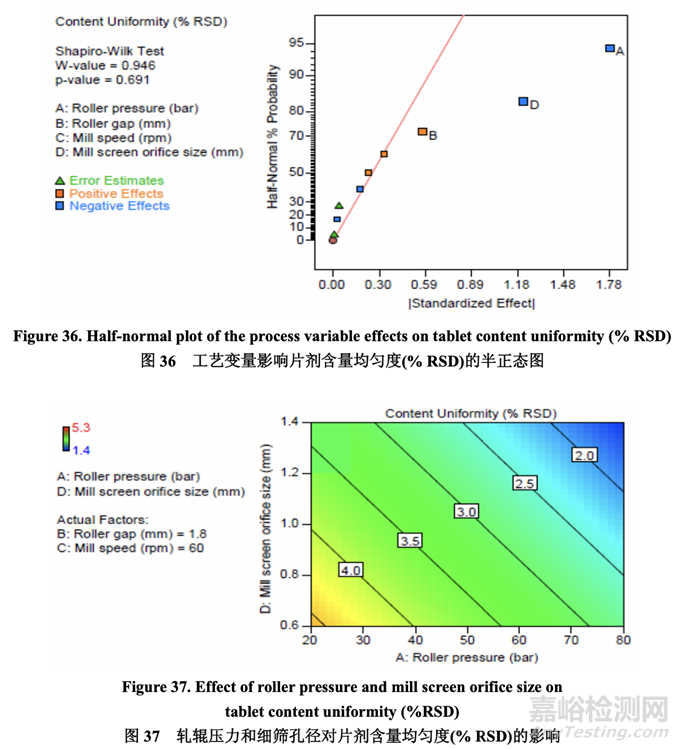
Since both ribbon density and tablet hardness were impacted by roller pressure and roller gap, it was logical to evaluate if any correlation existed between these two quality attributes. As shown in Figure 35, an inverse relationship was observed between ribbon density and tablet hardness. The establishment of this relationship was significant as it enables an intermediate material attribute (ribbon density) to be used as an in-process control during roller compaction to facilitate successful downstream operation (tablet compression) and ensure the target for a final product quality attribute (dissolution) is met.
因?yàn)閹钗锩芏群推瑒┯捕榷际苘堓亯毫蛙堓侀g隙影響,所以評(píng)估這兩個(gè)質(zhì)量屬性間是否 存在任何相關(guān)性是合理的�����。如圖 35 所示�����,觀察到帶狀物密度和片劑硬度間有逆相關(guān)性。建 立該相關(guān)性是重要的因?yàn)樗苁怪虚g體物料屬性(帶狀物密度)在碾壓中作為在線控制使用 以方便下游運(yùn)行(壓片)成功并保證符合最終產(chǎn)品質(zhì)量屬性(溶出)的目標(biāo)�����。
Significant factors for tablet friability 片劑脆碎度的顯著因素
All tablets manufactured in Batch Nos. 37-47 exhibited acceptable friability (< 0.2% weight loss) when compressed using 10 kN and 15 kN of force. When 5 kN of compression force was used, Batch Nos. 41 and 44 exhibited low tablet hardness (< 5.0 kP) and failed the friability test. These two batches had high ribbon density (~ 1.28 g/cc). The remainder of the batches compressed using 5 kN of force showed acceptable friability (< 0.2% weight loss) and hardness was higher than 5.0 kP.
當(dāng)用10 kN和15 kN力壓縮時(shí)�����,生產(chǎn)的所有片劑,批號(hào)37~47�����,顯示出脆碎度合格(重量損失< 0.2%)。當(dāng)使用5 kN壓縮力時(shí)�����,批號(hào)41和44顯示出片劑硬度低(< 5.0 kP)�����,脆碎度實(shí)驗(yàn)不合格�����。 這兩批的帶狀物密度高(~ 1.28 g/cc)�����。用5 kN力壓縮的其余批次顯示出脆碎度合格(重量損失< 0.2%),硬度高于5.0 kP�����。
Significant factors for tablet assay 片劑含量的顯著因素
All batches demonstrated acceptable assay (ranging from 98.4-100.6%) which is well within the specification limits (95.0-105.0% w/w) and none of the factors showed a significant impact on tablet assay. 所有批都顯示出含量合格(范圍為98.4~100.6%)�����,處于質(zhì)量標(biāo)準(zhǔn)限度(95.0~105.0% w/w)內(nèi), 無(wú)因素顯示出對(duì)片劑含量有顯著影響�����。
Significant factors for tablet content uniformity (% RSD)
片劑含量均勻度(% RSD)的顯著因素
Data analysis indicated that the curvature effect was not significant for tablet content uniformity. As shown in the half-normal plot (Figure 36), the significant factors affecting tablet content uniformity were A (roller pressure), D (mill screen orifice size) and B (roller gap).
數(shù)據(jù)分析表明曲率效應(yīng)對(duì)片劑含量均勻度無(wú)意義�����。如半正態(tài)圖(圖36)所示,影響片劑含量均 勻度的顯著因素為A (軋輥壓力), D (細(xì)篩孔徑)和B (軋輥間隙)�����。
Figure 37 shows the effect of roller pressure and mill screen orifice size on tablet content uniformity. Tablet content uniformity improved as evidenced by a decreased % RSD with increasing roller pressure and mill screen orifice size. Roller gap had some impact on tablet content uniformity but to a lesser extent.
圖 37 顯示了軋輥壓力和細(xì)篩孔徑對(duì)片劑含量均勻度的影響�����。片劑含量均勻度隨軋輥壓力和 細(xì)篩孔徑的增加而提高�����,通過(guò)% RSD減少所證明�����。軋輥間隙對(duì)片劑含量均勻度有影響但程 度較輕。
Significant factors for tablet disintegration 片劑崩解的顯著因素
All batches demonstrated rapid disintegration (< 4 min). None of the process variables studied had a significant impact on the disintegration time.
所有批顯示出快速崩解(< 4 min)。無(wú)研究的工藝變量對(duì)崩解時(shí)間有顯著影響�����。
Significant factors for tablet dissolution 片劑溶出的顯著因素
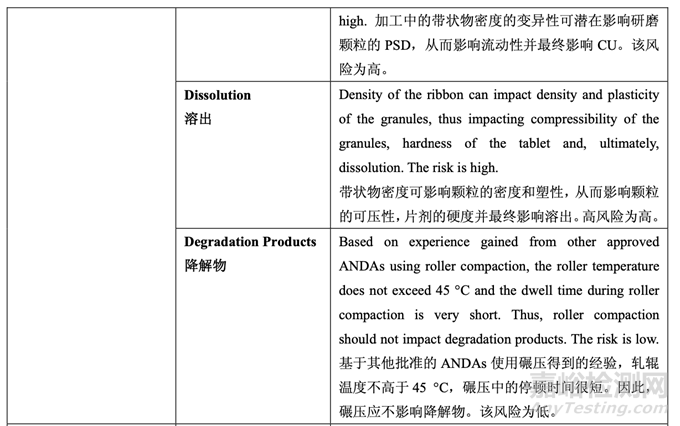
Tablet hardness had a significant impact on dissolution (see Section 2.3.5 Tablet Compression Process Development). Based on the inverse linear relationship between ribbon density and tablet hardness, it can be concluded that roller compaction will have an indirect impact on dissolution. For a ribbon with a reasonable density, target hardness can be achieved by adjusting the main compression force. However, it is well known that powder material loses a certain extent of its compressibility and compactability when roller compacted. Consequently, higher compression force is required to achieve the same tablet hardness for a higher ribbon density than for a lower ribbon density. On the other hand, when the ribbon density was low (≤ 1.0 g/cc), the flowability of the granules (Batches 2 and 3) was low (ffc < 6). Therefore, a range for ribbon density needs to be established such that the desired granule flowability is achieved and the required compression force will not exceed the maximum allowable tool tip pressure recommended by the tooling manufacturer. Based on the DOE results for tablet friability and granule flowability, the ribbon density will be controlled between 1.0-1.2 g/cc (i.e., ribbon relative density between 0.68-0.81; ribbon true density is 1.4803 g/cc in this study).
片劑硬度對(duì)溶出有顯著影響(見(jiàn)2.3.5節(jié)壓片工藝開(kāi)發(fā))?����;趲钗锩芏群推瑒┯捕乳g對(duì)逆線 性關(guān)系,可得出結(jié)論�����,碾壓將間接影響溶出。對(duì)于密度合理的帶狀物�����,通過(guò)調(diào)整主壓縮力來(lái) 達(dá)到目標(biāo)硬度�����。但是,眾所周知�����,粉末物料在碾壓時(shí)�����,會(huì)失去一定程度的壓縮性和壓實(shí)性�����。 因此�����,較高帶狀物密度比較低帶狀物密度,為達(dá)到相同的片劑硬度需較高的壓縮力�����。另一方 面�����,但帶狀物密度低(≤ 1.0 g/cc)時(shí)�����,顆粒(批2和3)的流動(dòng)性低(ffc < 6)�����。因此�����,需確定帶狀物 密度范圍以便達(dá)到預(yù)期的顆粒流動(dòng)性并且所需的壓縮力將不超過(guò)模具生產(chǎn)商建議允許的最 大工具提示壓力�����?����;谄瑒┐嗨槎群皖w粒流動(dòng)性的DOE結(jié)果�����,帶狀物密度應(yīng)控制在1.0-1.2 g/cc(即帶狀物相對(duì)密度為0.68~0.81;該研究中的帶狀物真密度為1.4803 g/cc)�����。
Summary of roller compaction and integrated milling process development
碾壓和集成粉碎工藝開(kāi)發(fā)的總結(jié)
Roller pressure had a significant impact on ribbon density, mean granule size (d50), granule flowability, tablet hardness and tablet content uniformity. Increasing roller pressure increased ribbon density, granule mean particle size (d50), granule flowability and tablet content uniformity (lower % RSD). However, it had a negative impact on the compressibility and compactability of the granules as indicated by decreasing tablet hardness for any given compression force.
軋輥壓力對(duì)帶狀物密度�����、顆粒平均粒徑(d50)、顆粒流動(dòng)性�����、片劑硬度和片劑含量均勻度有 顯著影響。軋輥壓力增加�����,帶狀物密度�����、顆粒平均粒徑(d50)�����、顆粒流動(dòng)性和片劑含量均勻 度(% RSD較低)增加�����。但是�����,對(duì)任何給定的壓縮力�����,它對(duì)顆粒的壓縮性和壓實(shí)性有負(fù)影響�����, 如減少片劑硬度所示�����。
Roller gap exhibited a significant impact on ribbon density, granule flowability, tablet hardness and tablet content uniformity. Increasing the roller gap decreased ribbon density, granule flowability and tablet content uniformity (higher % RSD). However, tablet hardness at a given compression force increased with increasing roller gap. 軋輥間隙對(duì)帶狀物密度�����、顆粒流動(dòng)性�����、片劑硬度和片劑含量均勻度有顯著影響。軋輥間隙增 加�����,帶狀物密度�����、顆粒流動(dòng)性和片劑含量均勻度(% RSD較高)減少�����。但是�����,在給定壓縮力下�����, 片劑硬度隨軋輥間隙增加而增加�����。
Mill screen orifice size had a significant impact on mean granule size (d50), granule flowability and tablet content uniformity. Increasing mill screen orifice size increased granule mean particle size (d50), granule flowability and tablet content uniformity (lower % RSD). 細(xì)篩孔徑對(duì)顆粒平均粒徑(d50)�����、顆粒流動(dòng)性和片劑含量均勻度有顯著影響�����。細(xì)篩孔徑增加, 顆粒平均粒徑(d50)�����、顆粒流動(dòng)性和片劑含量均勻度(% RSD較低)增加。
Mill speed did not show a significant impact on any of the responses studied. In addition, no curvature effects were observed for any of the responses. Based on the results of the DOE study, roller pressure, roller gap and mill screen orifice size were identified as the CPPs while mill speed was determined to be not critical.
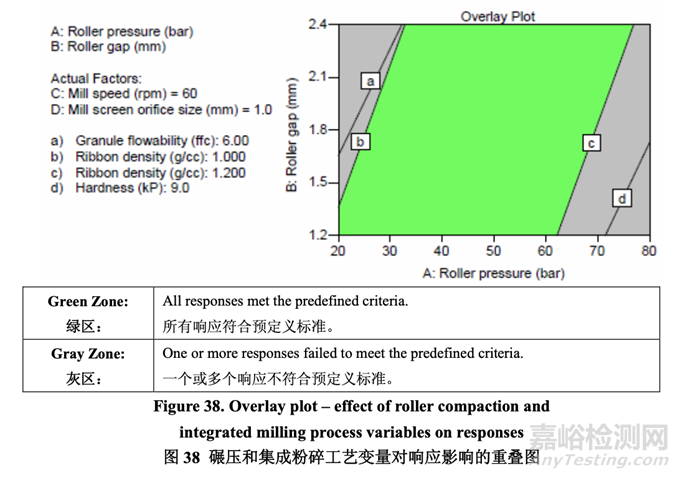
粉碎機(jī)速度對(duì)任何研究的響應(yīng)未顯示出有顯著影響�����。此外,任何響應(yīng)未觀察到曲率效應(yīng)�����?����;?于DOE研究的結(jié)果,當(dāng)確定粉碎機(jī)速度為非關(guān)鍵時(shí)�����,確定軋輥間隙和細(xì)篩孔徑為CPPs�����。 The overlay plot shown in Figure 38 was used to identify an appropriate range for each CPP that would ensure that the targets for all quality attributes are met concurrently. A mill screen orifice size of 1.0 mm was selected because it allows a wider acceptable operating range for both roller pressure and roller gap compared to the other studied sizes (0.6 mm and 1.4 mm). Based on the results, the acceptable ranges for roller pressure and roller gap were identified as 20-77 bar and 1.2-2.4 mm, respectively, for the roller compaction and integrated milling process step using an Alexanderwerk WP120 equipped with a knurled roller that is 120 mm in diameter and 25 mm in width.
圖 38 所示的重疊圖用于確定每個(gè) CPP 的合適范圍,可保證所有質(zhì)量屬性都同時(shí)符合其目標(biāo)�����。 選擇細(xì)篩孔徑為 1.0 mm�����,因?yàn)橄啾扔谄渌芯康目讖?0.6 mm 和 1.4 mm)�����,它提供了軋輥壓 力和軋輥間隙一個(gè)較寬的可接受操作范圍�����?����;诮Y(jié)果�����,對(duì)于使用裝備滾花輥(直徑為 120 mm 和寬為25 mm)的Alexanderwerk WP120的碾壓和集成粉碎工藝步驟�����,確定了軋輥壓力和軋 輥間隙可接受范圍分別為 20~77 bar 和 1.2~2.4 mm�����。
Updated Risk Assessment of the Roller Compaction and Integrated Milling Process Variables
更新的碾壓和集成粉碎工藝變量的風(fēng)險(xiǎn)評(píng)估
Table 41 presents the risk reduction for the roller compaction and integrated milling process variables as a result of the development studies. Justification of the reduced risks is also provided. 表 41 呈現(xiàn)了由于開(kāi)發(fā)研究�����,降低了碾壓和集成粉碎工藝變量的風(fēng)險(xiǎn)�����。也提供了風(fēng)險(xiǎn)降低的 依據(jù)�����。
參考文獻(xiàn):
Example QbD IR Tablet Module 3 Quality 3.2.P.2 Pharmaceutical Development,F(xiàn)DA�����,2012.