This is an example pharmaceutical development report illustrating how ANDA applicants can move toward implementation of Quality by Design (QbD). The purpose of the example is to illustrate the types of pharmaceutical development studies ANDA applicants may use as they implement QbD in their generic product development and to promote discussion on how OGD would use this information in review.
FDA官網(wǎng)中一個有關(guān)藥物開發(fā)報告的實例,用以說明申請人如何實施質(zhì)量源于設(shè)計(QbD)����。 該實例的目的是說明ANDA申請人在其仿制藥開發(fā)過程中實施QbD時,可使用的藥物開發(fā)研究的類型����,同時促進探討OGD在審評中如何使用該信息。
本文主要概述了制劑的處方開發(fā)關(guān)鍵要點和一個處方開發(fā)案例����。
2.3.4 Final Blending and Lubrication Process Development 最終混合和潤滑工藝開發(fā)
Initial Risk Assessment of the Final Blending and Lubrication Process Variables 最終混合和潤滑工藝變量的初始風(fēng)險評估
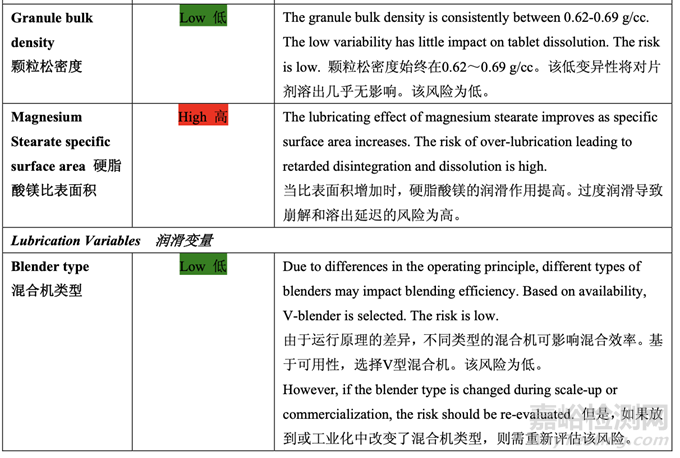
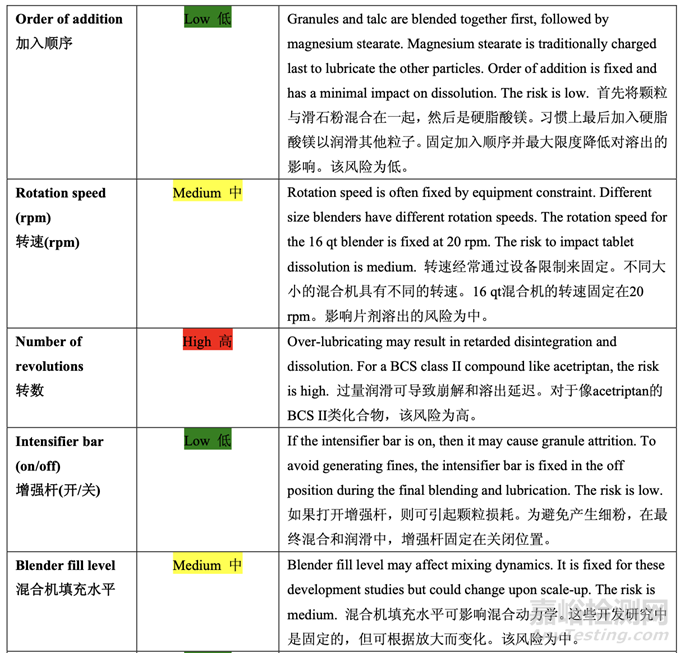
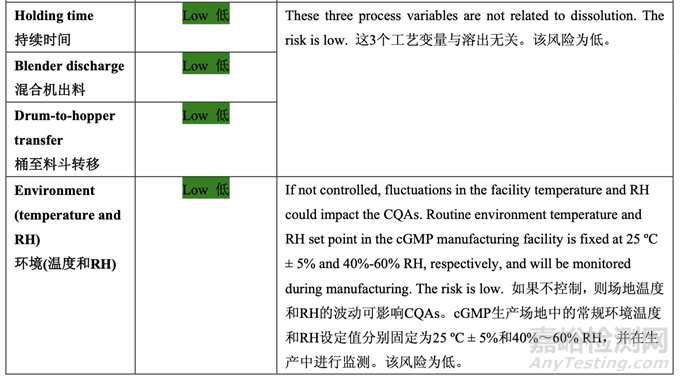
The initial risk assessment of the overall manufacturing process presented in Table 32 identified the risk of the final blending and lubrication step to impact tablet dissolution as high. The lubrication process variables that could potentially impact tablet dissolution were identified and their associated risk was evaluated. Table 42 presents the initial risk assessment of the final blending and lubrication step.
表 32 呈現(xiàn)的整體生產(chǎn)工藝的初始風(fēng)險評估確定了最終混合和潤滑步驟為影響片劑溶出的高 風(fēng)險。確定了可潛在影響片劑溶出的潤滑工藝變量并評估了它們相關(guān)的風(fēng)險�����。表 42 呈現(xiàn)了 最終混合和潤滑步驟的初始風(fēng)險評估��。
Based on the results of Formulation Development Study #2, the extragranular magnesium stearate and talc levels were fixed to 0.6% and 2.9%, respectively. The composition of Generic Acetriptan Tablets, 20 mg, was shown previously in Table 29.
基于處方開發(fā)研究#2 的結(jié)果��,外加硬脂酸鎂和滑石粉濃度分別固定為 0.6%和 2.9%�����。仿制藥 20 mg Acetriptan 片的組分如之前的表 29 所示��。
Due to the low solubility of acetriptan, it is important to ensure that the blend is notover-lubricated, leading to retarded disintegration. NIR monitoring of the lubrication process isnot feasible due to the low amount of lubricant added; therefore, a traditional method with the blending endpoint based on lubrication time is needed
由于acetriptan的溶解度低��,重要的是保證不過度潤滑混合物����,導(dǎo)致崩解延遲。NIR監(jiān)測潤滑 工藝是行不通的����,由于加入的潤滑劑數(shù)量少;因此,需要使用基于潤滑時間的混合終點的傳 統(tǒng)方法��。
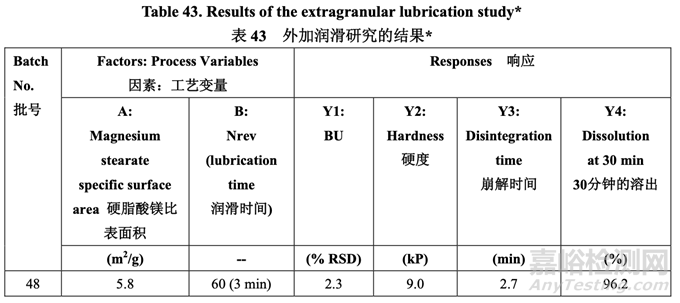
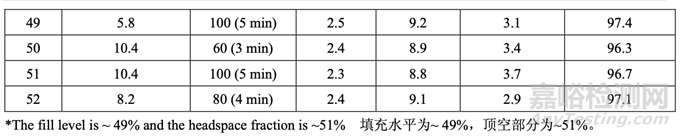
A study was performed to investigate the effect of magnesium stearate specific surface area and number of revolutions during lubrication on tablet hardness, disintegration, and dissolution. For this study, a 25.0 kg blend was manufactured in a pilot scale blender (150 L) using acetriptan Lot #2. The blend was roller compacted to give a ribbon relative density of 0.75. The ribbon was then milled and subdivided into five 5.0 kg batches. For each batch, the granules and talc were blended for 100 revolutions in a 16 qt V-blender at 20 rpm prior to lubrication with magnesium stearate. Then, magnesium stearate was added and blended according to the experimental design as shown in Table 43. After lubrication, samples were pulled from the 10 locations shown in Figure 23 to verify blend uniformity. The lubricated blend was then compressed using 10 kN of force to manufacture tablets. Ejection force was monitored. Compressed tablets were checked for appearance and the tablet press tooling (punches and dies) was evaluated for evidence of picking/sticking and binding. Additionally, tablets were subjected to friability, assay and content uniformity testing. Table 43 shows the lubrication parameters and results for each batch (not all data shown).
進行了一項研究以研究潤滑中的硬脂酸鎂比表面積和轉(zhuǎn)數(shù)對片劑硬度�����,崩解和溶出的影響����。 該研究,使用 acetriptan 批號 2 在中試規(guī)?�;旌蠙C(150 L)中生產(chǎn)了 25.0 kg 混合物�����。碾壓混合 物以產(chǎn)生帶狀物相對密度為 0.75。然后粉碎帶狀物����,細分為各 5.0 kg 的 5 批。對于每批��,在 與硬脂酸鎂潤滑前��,在 20 rpm 轉(zhuǎn)速的 16 qt V 型混合機中��,顆粒與滑石粉混合 100 轉(zhuǎn)數(shù)����。 然后,根據(jù)如表 43 所示的實驗設(shè)計加入硬脂酸鎂并混合����。潤滑后,從如圖 23 所示的 10 個 位置取樣以驗證混合均勻����。然后使用 10 kN 力壓縮潤滑后的混合物來生產(chǎn)片劑。監(jiān)測脫模力����。 檢查壓制片的外觀,評估壓片機模具(沖頭和沖模)粘沖和粘附的證據(jù)��。此外�����,片劑進行脆碎 度��,含量和含量均勻度檢查����。表 43 顯示了每批的潤滑參數(shù)和結(jié)果(未顯示所有數(shù)據(jù))。
The ejection force increased slightly with decreased lubrication time and lower specific surfacearea but did not exceed 150 N during the study. Tablet elegance was not an issue as allcompressed tablets had a smooth surface and lacked any visible striations on the sides of the tablet. There was no evidence of product sticking on the punches within the letters and numbers. There was also no evidence of binding to the die cavities.
當潤滑時間減少和比表面積降低時��,脫模力略微增加����,但在研究中不超過150 N。片劑美觀 度不是問題因為所有的壓制片表面光滑����,片劑兩端無任何可見的條紋。在字母和數(shù)字范圍內(nèi)��, 無產(chǎn)品粘沖的證據(jù)��。也沒有與模腔粘附的證據(jù)。
For each batch, the % RSD was less than 3% indicating that blend uniformity was acceptable following lubrication of the granules. Overall, the blend assay was between 98.3% and 101.7% for all samples pulled during the study. The tablet hardness observed was 9.0 ± 0.2 kP which is well within the target range of 8.0-10.0 kP. Tablets exhibited rapid disintegration (< 4 min) and dissolution (> 95% in 30 min). The results indicated that adequate lubrication of the granules was insensitive to both specific surface area (5.8-10.4 m2/g) and lubrication time (3-5 min) within the ranges studied.
對于每批����,% RSD 低于 3%表明顆粒潤滑后,混合物均勻度是合格的��?�?傮w而言����,研究中取 樣的所有樣品的混合物含量為 98.3%~101.7%。觀察到的片劑硬度為 9.0 ± 0.2 kP����,位于目標 范圍 8.0~10.0 kP 內(nèi)。片劑顯示出快速崩解(< 4 min)和溶出(> 95%�����,30 min)����。結(jié)果表明潤滑 適度的顆粒對研究范圍內(nèi)的比表面積(5.8~10.4 m2/g)和潤滑時間(3~5 min)都不敏感。
Over the course of the study, friability did not exceed 0.2% w/w. Tablet assay was close to target and well within the acceptable range of 95.0-105.0% w/w. Tablet content uniformity was acceptable with a % RSD less than 4%.
在研究過程中,脆碎度不超過0.2% w/w��。片劑含量接近于目標��,處于合格范圍95.0~105.0% w/w內(nèi)����。片劑含量均勻度合格��,其% RSD低于4%��。
Summary of Final Blending and Lubrication Process Development
最終混合和潤滑工藝開發(fā)的總結(jié)
Within the ranges studied, magnesium stearate specific surface area (5.8-10.4 m2/g) and number of revolutions (60-100) did not have a significant impact on the drug product quality attributes studied.
在研究的范圍內(nèi)�����,硬脂酸鎂比表面積(5.8~10.4 m2/g)和轉(zhuǎn)數(shù)(60~100)對研究的制劑質(zhì)量屬性 無顯著影響�����。
Updated Risk Assessment of the Final Blending and Lubrication Process Variables
更新的最終混合和潤滑工藝變量的風(fēng)險評估
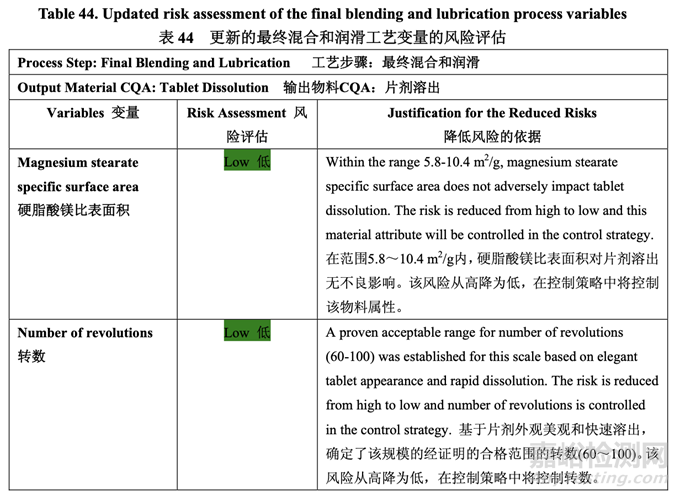
Table 44 presents the risk reduction for the final blending and lubrication step as a result of the development studies. Only the process variables that were initially identified as high risk to the dissolution of the final drug product are shown.
表 44 呈現(xiàn)了由于開發(fā)研究�����,降低了最終混合和潤滑步驟的風(fēng)險��。僅顯示了初始確定為最終 制劑溶出高風(fēng)險的工藝變量。
2.3.5 Tablet Compression Process Development
壓片工藝開發(fā)
Initial Risk Assessment of the Tablet Compression Process Variables
壓片工藝變量的初始風(fēng)險評估
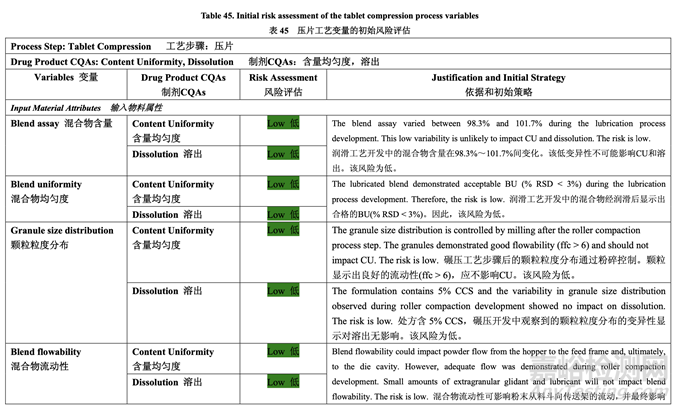
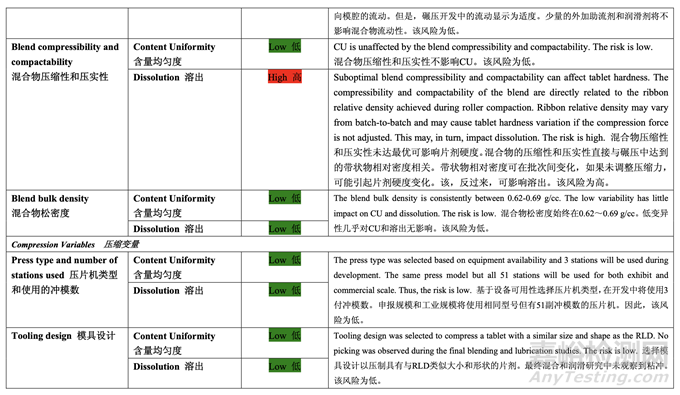
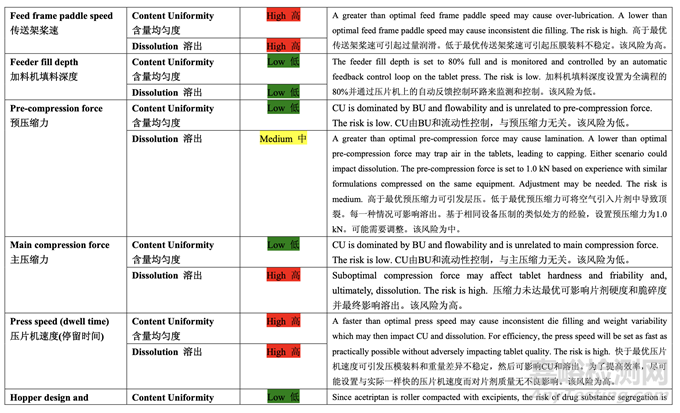
Based on the initial risk assessment of the overall manufacturing process shown in Table 32, the risk of the compression step to impact content uniformity and dissolution of the tablets was identified as high. Process variables that could potentially impact these two drug product CQAs were identified and their associated risk was evaluated. The results of the initial risk assessment of the compression process variables are summarized in Table 45.
基于整體生產(chǎn)工藝的初始風(fēng)險評估����,如表 32 所示,確定壓縮步驟為影響片劑含量均勻度和 溶出的高風(fēng)險�����。確定了可潛在影響這兩個制劑 CQAs 的工藝變量并評估了它們的相關(guān)風(fēng)險��。 表 45 總結(jié)了壓縮工藝變量的初始風(fēng)險評估的結(jié)果��。
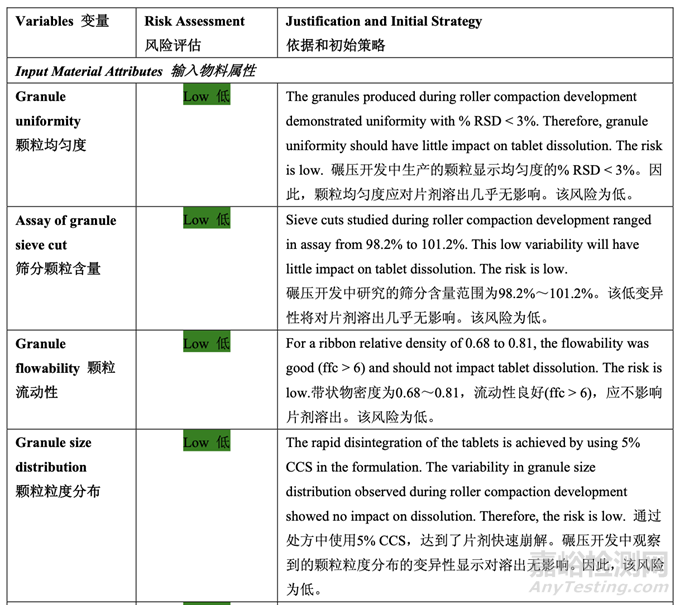
The following experiments were undertaken to investigate the relationship between the input material attributes (i.e., ribbon relative density) and process parameters related to compression and the final drug product quality attributes. Three batches of final blend (Batch No. 53-55, 15.0 kg each, drug substance Lot #2) were manufactured in a 50 L blender for the compression studies. The ribbon relative density for these three batches was 0.68, 0.75 and 0.81, respectively. The roller compaction studies concluded that within this range, the necessary compression force will not exceed the maximum allowable tool tip pressure recommended by the manufacturer.
進行了如下實驗以研究輸入物料屬性(即帶狀物相對密度)和與壓縮相關(guān)的工藝參數(shù)及輸出 制劑質(zhì)量屬性間的關(guān)系����。使用壓縮研究中的50 L混合機生產(chǎn)了3批最終混合物(批號53~55, 15.0 kg每批,原料藥批號2)��。這3批的帶狀物相對密度分別為0.68, 0.75和0.81��。碾壓研究推 斷出在該范圍內(nèi)����,必要的壓縮力將不超過生產(chǎn)商推薦的容許的最大工具提示壓力。
Effect of Feeder Frame Paddle Speed 傳送架槳速的影響
A screening study to investigate the impact of the feeder frame paddle speed (8-20 rpm) on tablet quality attributes was conducted. Since the final blend flows well, changes in feeder frame paddle speed within the specified range had no impact on tablet weight variability or content uniformity. Tablet dissolution was also unaffected by changes in feeder speed, suggesting that over-lubrication due to the additional mixing is not a concern. This process variable was eliminated from further study.
為研究傳送架槳速(8~20 rpm)對片劑質(zhì)量屬性的影響而進行了一項篩查研究�����。因為最終混合 物流動性好,在規(guī)定范圍內(nèi)改變傳送架槳速不影響片重差異或含量均勻度����。改變加料機速度 也不影響片劑溶出����,表明由于額外混合的過量潤滑不是問題。進一步研究排除了該工藝變量��。
Effect of Main Compression Force, Press Speed and Ribbon Relative Density 主壓縮力����,壓片機速度和帶狀物相對密度的影響
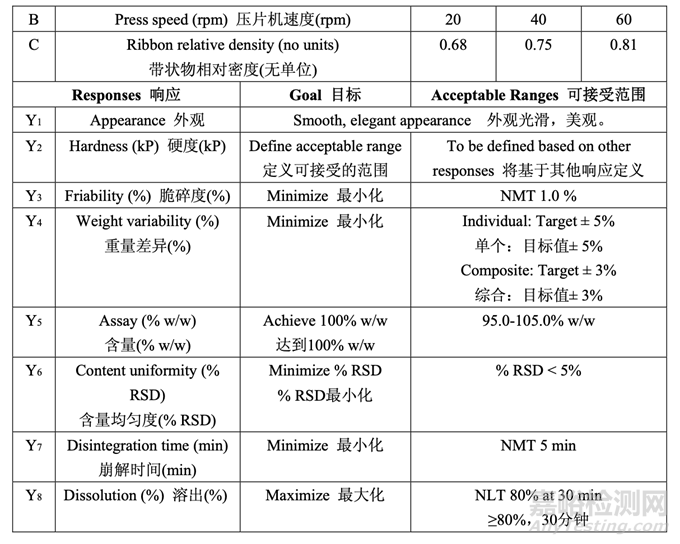
Compression force and press speed (which is related to dwell time) can affect numerous quality attributes including hardness, disintegration, dissolution, assay, content uniformity, friability, weight variability and appearance. The density of the ribbon following roller compaction may also impact the compressibility and compactability of the granules which would then impact tablet hardness and dissolution. Therefore, a 23 full factorial DOE with three center points was performed to understand the effects of these parameters on tablet quality attributes. Pre-compression force is important to reduce entrapped air that can impact the tablet integrity. However, based on previous experience with similar formulations compressed with similar tooling (ANDA 123456), the pre-compression force was fixed to 1 kN for this DOE. Table 46 presents the study design and acceptance criteria for the responses.
壓縮力和壓片機速度(與停頓時間相關(guān))可影響許多質(zhì)量屬性包括硬度,崩解����,溶出,含量����, 含量均勻度,脆碎度�����,重量差異和外觀。碾壓后的帶狀物密度也可影響顆粒的壓縮性和壓實 性��,然后影響片劑硬度和溶出��。因此����,進行了帶 3 個中心點的 23 全因素 DOE 以了解這些參 數(shù)對片劑質(zhì)量屬性的影響。預(yù)壓縮力降低可影響片劑完整性的包埋空氣是重要的��。但是����,基 于用類似模具壓制的類似處方(ANDA 123456)的先前經(jīng)驗,固定該 DOE 的預(yù)壓縮力為 1 kN����。 表 46 呈現(xiàn)了研究設(shè)計和響應(yīng)的可接受標準。
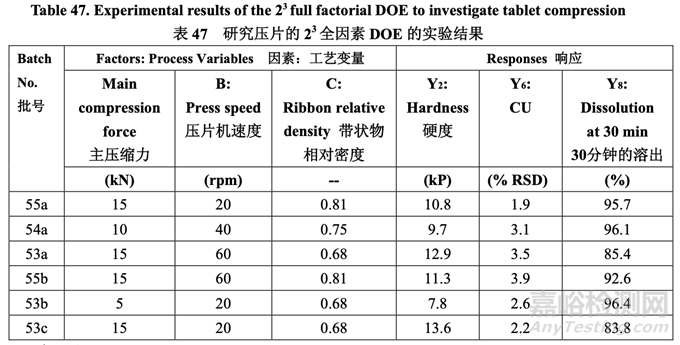
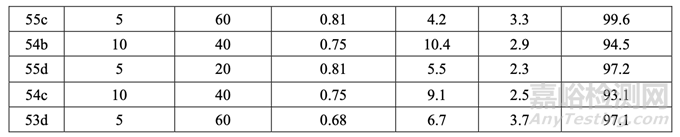
The press was run at the speed of the specified DOE for at least five minutes prior to any sampling. Tablet samples were then pulled at the beginning, middle and end of each run (except for Batch No. 54c which was sampled every 20 min throughout the entire run). Similar responses were observed at each sample time point; therefore, Table 47 presents the results for the middle time point (responses Y1, Y3, Y4, Y5 and Y7 not shown).
壓片機以規(guī)定的 DOE 速度運行至少 5 分鐘后����,進行取樣。在每批片劑(批號 54c 除外����,該批 每隔 20 分鐘取樣)的開始����,中間和末端取樣����。樣品的每個時間點觀察到類似的響應(yīng);因此, 表 47 呈現(xiàn)了中間時間點的結(jié)果(響應(yīng) Y1, Y3, Y4, Y5 和 Y7 未顯示)��。
Significant factors for tablet hardness 片劑硬度的顯著因素
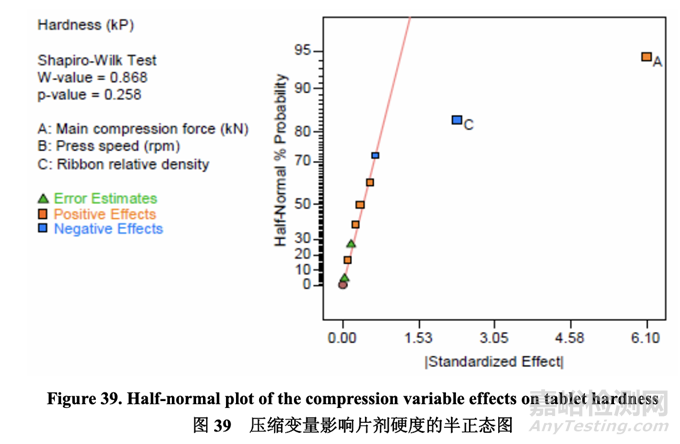
Since center points were included in the study design, the significance of the curvature effect was tested using an adjusted model and was found to be not significant. Thus, center points were included for model fitting. As shown in the following half-normal plot (Figure 39), A (main compression force) was the dominating factor affecting tablet hardness followed by C (ribbon relative density). The remaining model terms had no significant impact because they came from the normally distributed population as pure error based on Shapiro-Wilk hypothesis test results.
因為中心點包括在研究設(shè)計中�����,所以使用校正模型檢驗了曲率效應(yīng)的意義����,發(fā)現(xiàn)無意義����。因 此,中心點包括了模型擬合��。如以下半正態(tài)圖(圖 39)所示�����,A (主壓縮力)為影響片劑硬度的 主要因素,其次是 C (帶狀物相對密度)��。剩余的模型項無顯著影響因為基于 Shapiro-Wilk 假 設(shè)檢驗結(jié)果�����,它們來自于作為純誤差的正態(tài)分布群體��。
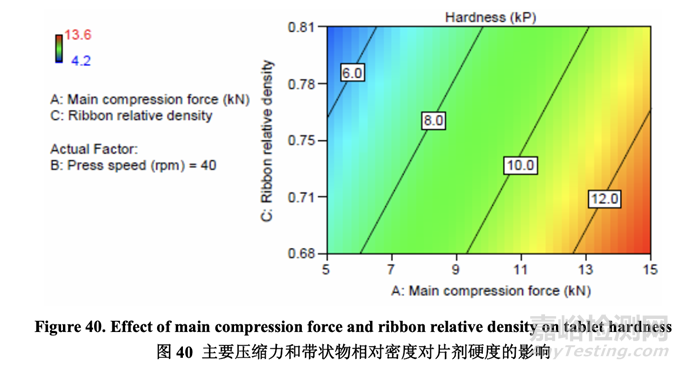
Tablet hardness was directly related to main compression force and inversely related to ribbon relative density as shown in the contour plot below (Figure 40). Both the half-normal plot and the contour plot show that there was no interaction between these two factors.
片劑硬度直接與主壓縮力相關(guān)����,與帶狀物相對密度密切相關(guān),如下面等高圖(圖40)所示����。半 正態(tài)圖和等高圖都顯示這兩個因素間無交互作用。
A roller compacted ribbon that exhibits a relative density toward the upper end of the acceptable range (0.81) required a greater compression force to achieve the same hardness than ribbon with a relative density toward the lower end of the acceptable range (0.68). This is because the powder mixture loses some of its compressibility and compactability after roller compaction.
顯示相對密度接近可接受范圍上端(0.81)的碾壓帶狀物比相對密度接近可接受范圍下端(0.68) 的帶狀物����,需要較大的壓縮力以達到相同的硬度。這是因為粉末混合物在碾壓后失去了其某 些壓縮性和壓實性�����。
The DOE results show that it is possible to adjust a process parameter to accommodate variability in a material attribute. In other words, the model can be used to determine the necessary compression force for a given ribbon relative density to ensure that the target tablet hardness is achieved.
DOE結(jié)果表明調(diào)整工藝參數(shù)以適應(yīng)物料屬性的變異性是可能的。換句話說�����,該模型可用于決 定給定帶狀物相對密度必要的壓縮力以保證達到片劑目標硬度����。
Significant factors for tablet friability 片劑脆碎度的顯著因素
None of the factors had a significant effect on tablet friability. All of the batches showed friability less than 0.2% except for Batch No. 55c which had an average hardness of 4.2 kP and showed a higher weight loss of 0.6%. Therefore, the lower limit for tablet hardness was set to 5.0 kP. 無因素對片劑脆碎度有顯著因素。所有批除批號55c外�����,都顯示出脆碎度低于0.2%��,批號55c 的平均硬度為4.2 kP并顯示出較高的重量損失����,為0.6%�����。因此��,片劑硬度的下限設(shè)置為5.0 kP�����。
Significant factors for tablet weight variability and content uniformity 片重差異和含量均勻度的顯著因素
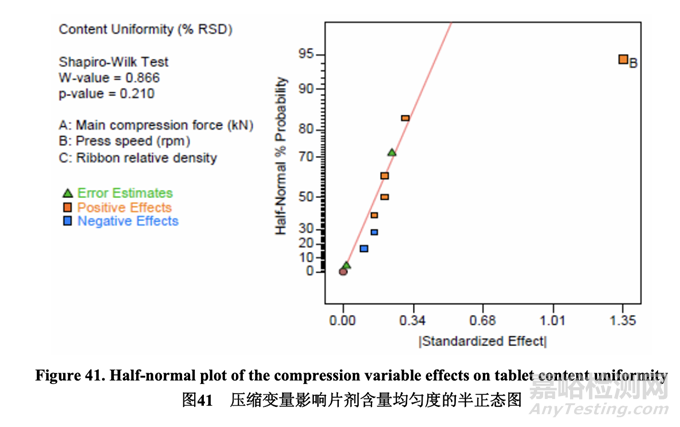
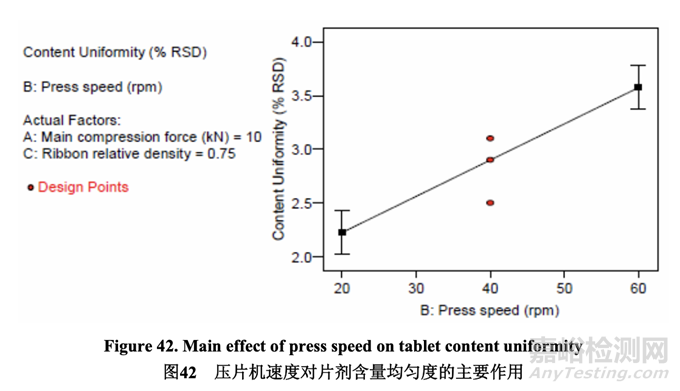
The half-normal plot below (Figure 41) shows that press speed was the only factor that had a significant impact on content uniformity. The effect was a positive effect, meaning that the % RSD increased as press speed increased. This is also shown clearly in the main effect plot (Figure 42). The main effect plot demonstrates that no curvature was observed so further optimization of the press speed is unnecessary.
如下的半正態(tài)圖(圖 41)表明,壓片機速度是顯著影響含量均勻度的唯一因素��。該作用為正作 用��,意味著% RSD 隨壓片機速度增加而增加��。這在主要作用圖(圖 42)中也得到清楚地顯示�����。 主要作用圖顯示未觀察到曲率����,因此壓片機速度無需進一步優(yōu)化。
Although better content uniformity (i.e., lower % RSD) is achieved when the tablet press is operated at a slower speed, the press speed range investigated (20-60 rpm) did not result in out-of-specification tablet content uniformity. At 60 rpm, the % RSD observed was less than 4% and well below the limit of 5%.
雖然壓片機以較慢速度運行時可得到較好的含量均勻度(即較低的% RSD)����,但是研究的壓片 機速度范圍(20~60 rpm)不會導(dǎo)致片劑含量均勻度超出質(zhì)量標準。在60 rpm�����,觀察到的% RSD 低于4%��,低于限度5%。
Similarly, press speed had a statistically significant impact on tablet weight variability which increased with faster press speed. However, the individual tablet weight variability was well below 5% and the composite weight variability was well below 3%.
類似地����,壓片機速度對片重差異的影響具有統(tǒng)計學(xué)意義,隨壓片機速度增加而增加����。但是, 單個片重差異低于 5%�����,綜合片重差異低于 3%��。
During production, it is desirable to maximize efficiency by setting the tablet press as fast as practically possible without adversely impacting the quality of the drug product. Based on the compression study, the proven acceptable range for press speed is 20-60 rpm.
生產(chǎn)中�����,通過盡可能設(shè)置與實際一樣快的壓片機速度而對片劑質(zhì)量無不良影響來使效率最大 化是可行的����?���;趬嚎s研究�����,壓片機速度已證明的可接受范圍是20~60 rpm�����。
Significant factors for tablet disintegration and dissolution 片劑崩解和溶出的顯著因素
The main compression force, press speed, and ribbon relative density did not have a significant impact on disintegration. The disintegration time was rapid and varied from 1.5 minutes to 3 minutes.
主壓縮力����,壓片機速度和帶狀物相對密度對崩解無顯著影響��。崩解時間快速�����,從1.5分鐘至3 分鐘變化����。
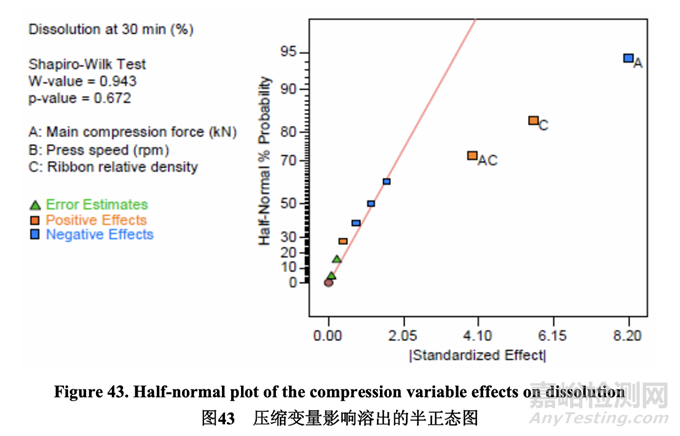
The following half-normal plot (Figure 43) shows that the significant factors affecting the dissolution rate of the compressed tablets were A (main compression force) and C (ribbon relative density). These two factors also showed a significant interaction, AC. The remaining model terms had no significant impact based on Shapiro-Wilk hypothesis test results.
如下的半正態(tài)圖(圖 43)表明,影響壓制片溶出率的顯著因素為 A (主壓縮力)和 C (帶狀物相 對密度)�����。這兩個因素也顯示出顯著的交互作用,AC����。基于 Shapiro-Wilk 假設(shè)檢驗結(jié)果�����,剩 余模型項無顯著影響����。
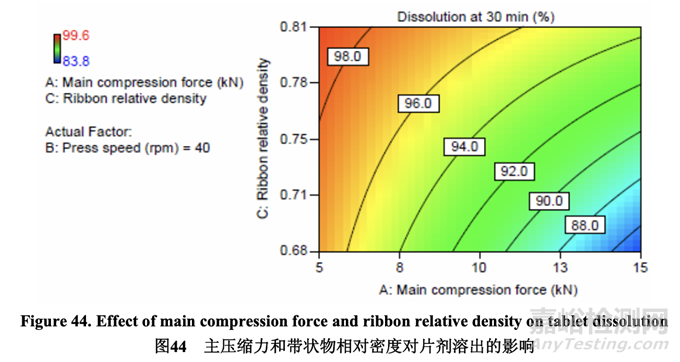
Figure 44 illustrates the effect of main compression force and ribbon relative density on tablet dissolution. The curved contour lines show that an interaction exists because the dissolution results differed depending on the main compression force setting and the ribbon relative density. The dissolution rate decreased with increasing main compression force and increased with increasing ribbon relative density. These results are in line with the observed effect that these factors had on tablet hardness. Increasing the main compression force resulted in harder tablets and retarded dissolution even though rapid disintegration was still achieved by using 5% superdisintegrant. To avoid a potential dissolution failure, the upper limit for hardness is set to 13.0 kP since Batch No. 53c with a hardness of 13.6 kP showed dissolution of 83.8%.
圖 44 說明了主壓縮力和帶狀物相對密度對片劑溶出的影響。等高曲線表明存在交互作用�����, 因為溶出結(jié)果隨主壓縮力設(shè)置和帶狀物相對密度而不同��。溶出率隨主壓縮力增加而減少��,隨 帶狀物相對密度增加而增加��。這些結(jié)果與這些因素對片劑硬度觀察到的影響一致��。主壓縮力 增加導(dǎo)致片劑硬度增加�����,溶出延遲����,即使通過使用 5%超級崩解劑仍然達到了快速崩解。為 避免潛在的溶出不合格����,硬度的上限設(shè)置為 13.0 kP 因為硬度為 13.6 kP 的批號 53c 顯示出溶 出為 83.8%。
Effect of compression run time on tablet weight variability
壓縮運行時間對片重差異的影響
Batch No. 54c was sampled every 20 minutes to evaluate the potential drift in tablet weight over the course of the compression run. The results demonstrated that the weight variability was well controlled for the individual tablets within ± 5% of the target weight and for the composite sample within ± 3% of the target weight. No trend for tablet weight was observed throughout the entire compression run. Tablet samples pulled at the beginning, middle, and end of the run were tested for all DOE responses and results are shown in Table 47. 批號54c每隔20分鐘取樣以評估壓縮運行過程中����,片重的潛在漂移。結(jié)果表明重量差異可控�����, 單個片劑在目標重量的± 5%內(nèi)�����,綜合樣品在在目標重量的± 3%內(nèi)����。整個壓縮運行中��,未觀 察到片重的趨勢����。檢驗了在運行開始�����,中間和末端取樣的片劑樣品的所有DOE響應(yīng)��,結(jié)果見表47��。
Summary of other responses 其他響應(yīng)的總結(jié)
Main compression force, press speed, and relative ribbon density had no significant impact on the remaining responses. Each run produced tablets that had a smooth surface with no evidence of picking/sticking or capping. Assay ranged from 99.1% to 101.0%.主壓縮力�����,壓片機速度和帶狀物相對密度對剩余響應(yīng)無顯著影響�����。每次運行生產(chǎn)出的片劑具 有光滑表面��,無粘沖或頂裂跡象�����。含量范圍為99.1%~101.0%。
Summary of Tablet Compression Process Development 壓片工藝開發(fā)的總結(jié)
Within the range studied (8-20 rpm), feeder frame paddle speed did not impact the tablet dissolution. A press speed in the range of 20-60 rpm did not show any significant impact on the responses investigated. An acceptable range for compression force was identified. Force adjustments can be made to accommodate the acceptable variation in ribbon relative density (0.68-0.81) between batches.
在研究的范圍內(nèi)(8~20 rpm)�����,傳送架槳速不影響片劑溶出��。壓片機速度在20~60 rpm范圍內(nèi) 顯示對研究的響應(yīng)無任何顯著影響�����。確定了壓縮力的可接受范圍�����?���?烧{(diào)整壓縮力以適應(yīng)批間 帶狀物相對密度可接受的差異(0.68~0.81)��。
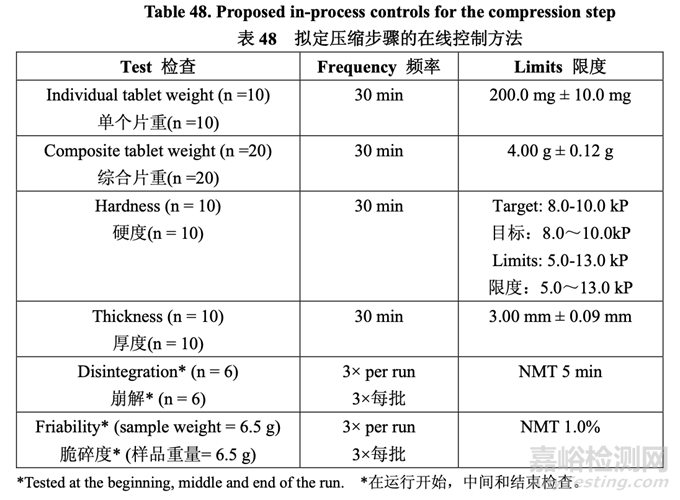
Proposed Tablet Compression In-Process Controls 擬定的壓片在線控制方法
Based on the results of the studies undertaken to understand the process variables affecting compression, Table 48 lists the proposed in-process controls for the compression step.
基于為理解影響壓縮的工藝變量而進行的研究結(jié)果����,表 48 列出了擬定壓縮步驟的在線控制 方法。
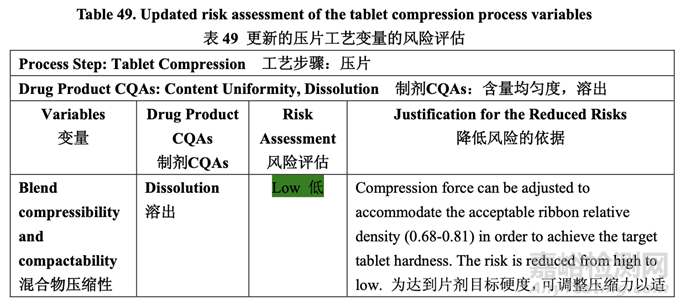
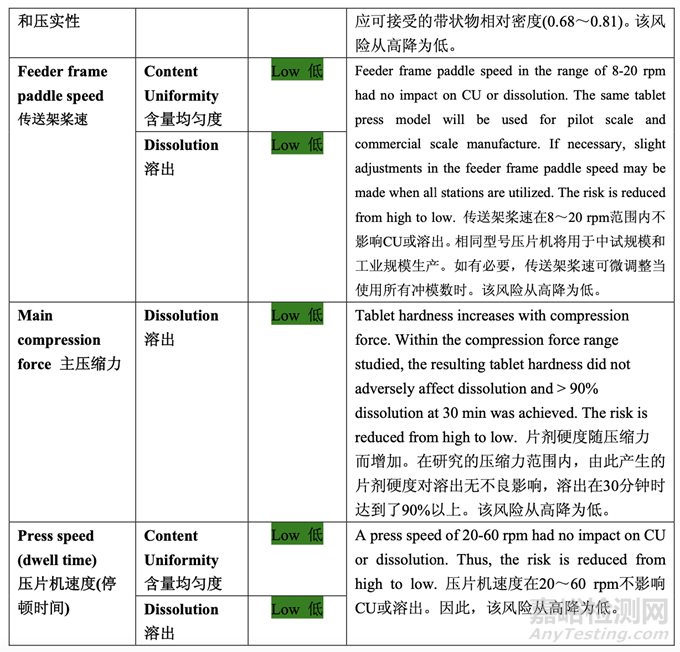
 Updated Risk Assessment of the Tablet Compression Process Variables
Updated Risk Assessment of the Tablet Compression Process Variables
更新的壓片工藝變量的風(fēng)險評估
The risks identified during the initial assessment of the compression step were reduced through development studies. The updated risk assessment is presented in Table 49.
整個開發(fā)研究中降低了在壓縮步驟初始評估期間確定的風(fēng)險�����。更新的風(fēng)險評估見表 49。
參考文獻:
Example QbD IR Tablet Module 3 Quality 3.2.P.2 Pharmaceutical Development�����,F(xiàn)DA��,2012.



 Updated Risk Assessment of the Tablet Compression Process Variables
Updated Risk Assessment of the Tablet Compression Process Variables