GS-2278的游離堿形式存在很多晶型,包括溶劑化物����,其鹽形式包括各種酸基���,綜合研究后,選擇鹽酸鹽二水合物的晶型�����。
特性鑒定
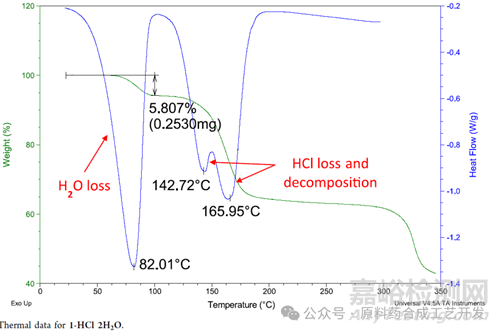
DSC-TGA1-HCl 2H2O is a dihydrated form (Karl Fischer =5.8% water, TGA weight loss = 5.8%) with a desolvation endotherm around 82 °C, followed by endotherms around 143 and 166 °C, attributed to HCl loss and decomposition, respectively.
DSC,熱���,三個(gè)吸熱峰,分別是失去水,失去氯化氫和分解��。
TGA�����,重,先失去水��,再失去氯化氫�,再分解,然后恒重�����。
單晶
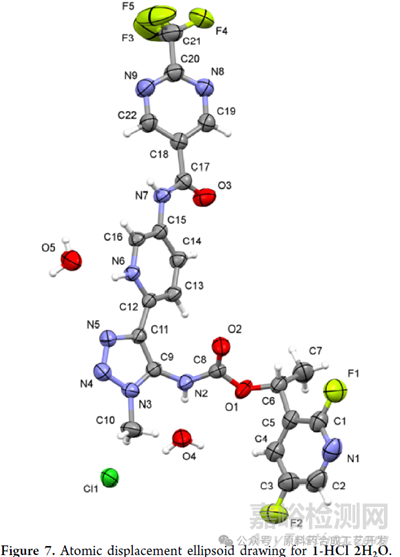
hydrochloric acid salt of 1, and it contains one freebase cation, one chloride anion, and two water molecules.
手性問題���,原文有�,這里沒有搬運(yùn)��,單晶是證明絕對構(gòu)型的最好證據(jù)�����。
穩(wěn)健性研究
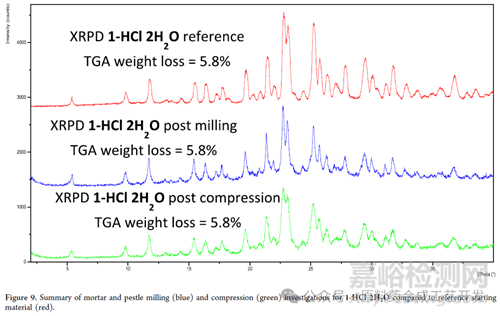
原料藥粉碎工序?qū)偷挠绊?/span>
As the clinical program progressed, a batch of 1-HCl 2H2O was jet-milled, and the resulting data confirmed that there was no change in crystalline form or water content
原料藥干燥工序?qū)偷挠绊?/span>
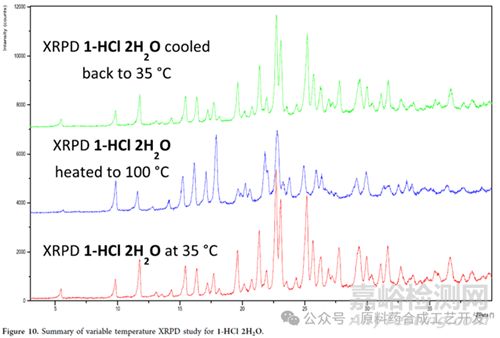
原料藥加熱到100度�,粉末衍射數(shù)據(jù)發(fā)生了變化,但是當(dāng)冷卻到35度時(shí)�����,粉末衍射數(shù)據(jù)和35度干燥的樣品粉末衍射數(shù)據(jù)一樣���,就是目標(biāo)晶型數(shù)據(jù)����。
數(shù)據(jù)說明,加熱條件水分會(huì)丟失�,晶型發(fā)生變化����,但是冷卻后缺水或者無水晶型會(huì)從環(huán)境中吸收水分變成2水合物���。
基于加熱����,溫度過高���,水分會(huì)丟失的風(fēng)險(xiǎn),干燥工序溫度未超過40度�。
生產(chǎn)環(huán)境濕度對原料藥水合物的影響
春夏秋冬���,不同時(shí)間點(diǎn)濕度不一樣�����,不一樣的車間濕度也不一樣�����。制劑生產(chǎn)過程����,原料藥暴露環(huán)境也涉及不同濕度環(huán)境���。
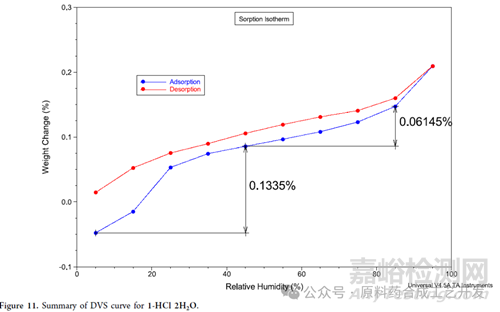
Dynamic vapor sorption (DVS) data can be used to gauge the robustness of a hydrate as a function of relative humidity conditions.
This indicates that 1-HCl
2H2O is resistant to desolvation/form change when exposed to nonhumid conditions and provides evidence of no conversion to a higher hydrate when exposed to very humid conditions.
參考文獻(xiàn)
https://doi.org/10.1021/acs.oprd.5c00065